Installing a Green Engine To Drive an Enzyme Cascade: A Light-Powered In Vitro Biosystem for Poly(3-hydroxybutyrate) Synthesis
Fei Li
Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, 32 West 7th Avenue, Tianjin Airport Economic Area, Tianjin, 300308 P. R. China
Search for more papers by this authorXinlei Wei
Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, 32 West 7th Avenue, Tianjin Airport Economic Area, Tianjin, 300308 P. R. China
Search for more papers by this authorLin Zhang
Key Laboratory of Plant Resources, Institute of Botany, Chinese Academy of Sciences, 20 Nanxincun, Xiangshan, Beijing, 100093 P. R. China
Search for more papers by this authorCheng Liu
Key Laboratory of Plant Resources, Institute of Botany, Chinese Academy of Sciences, 20 Nanxincun, Xiangshan, Beijing, 100093 P. R. China
Search for more papers by this authorProf. Dr. Chun You
Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, 32 West 7th Avenue, Tianjin Airport Economic Area, Tianjin, 300308 P. R. China
University of Chinese Academy of Sciences, 19A Yuquan Road, Shijingshan District, Beijing, 100049 P. R. China
National Technology Innovation Center of Synthetic Biology, 32 West 7th Avenue, Tianjin Airport Economic Area, Tianjin, 300308 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhiguang Zhu
Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, 32 West 7th Avenue, Tianjin Airport Economic Area, Tianjin, 300308 P. R. China
University of Chinese Academy of Sciences, 19A Yuquan Road, Shijingshan District, Beijing, 100049 P. R. China
National Technology Innovation Center of Synthetic Biology, 32 West 7th Avenue, Tianjin Airport Economic Area, Tianjin, 300308 P. R. China
Search for more papers by this authorFei Li
Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, 32 West 7th Avenue, Tianjin Airport Economic Area, Tianjin, 300308 P. R. China
Search for more papers by this authorXinlei Wei
Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, 32 West 7th Avenue, Tianjin Airport Economic Area, Tianjin, 300308 P. R. China
Search for more papers by this authorLin Zhang
Key Laboratory of Plant Resources, Institute of Botany, Chinese Academy of Sciences, 20 Nanxincun, Xiangshan, Beijing, 100093 P. R. China
Search for more papers by this authorCheng Liu
Key Laboratory of Plant Resources, Institute of Botany, Chinese Academy of Sciences, 20 Nanxincun, Xiangshan, Beijing, 100093 P. R. China
Search for more papers by this authorProf. Dr. Chun You
Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, 32 West 7th Avenue, Tianjin Airport Economic Area, Tianjin, 300308 P. R. China
University of Chinese Academy of Sciences, 19A Yuquan Road, Shijingshan District, Beijing, 100049 P. R. China
National Technology Innovation Center of Synthetic Biology, 32 West 7th Avenue, Tianjin Airport Economic Area, Tianjin, 300308 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhiguang Zhu
Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, 32 West 7th Avenue, Tianjin Airport Economic Area, Tianjin, 300308 P. R. China
University of Chinese Academy of Sciences, 19A Yuquan Road, Shijingshan District, Beijing, 100049 P. R. China
National Technology Innovation Center of Synthetic Biology, 32 West 7th Avenue, Tianjin Airport Economic Area, Tianjin, 300308 P. R. China
Search for more papers by this authorGraphical Abstract
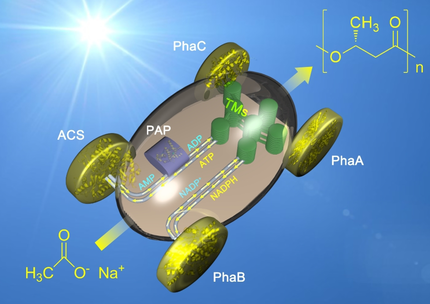
Thylakoid membranes were introduced into an in vitro biosystem for the synthesis of polyhydroxybutyrate to enable the conversion of light energy into biological energy through the regeneration of NADPH and ATP (see picture). The use of thylakoid membranes as a green engine in this way is a promising strategy for light-powered cell-free biomanufacturing.
Abstract
Many existing in vitro biosystems harness power from the chemical energy contained in substrates and co-substrates, and light or electric energy provided from abiotic parts, leading to a compromise in atom economy, incompatibility between biological and abiotic parts, and most importantly, incapability to spatiotemporally co-regenerate ATP and NADPH. In this study, we developed a light-powered in vitro biosystem for poly(3-hydroxybutyrate) (PHB) synthesis using natural thylakoid membranes (TMs) to regenerate ATP and NADPH for a five-enzyme cascade. Through effective coupling of cofactor regeneration and mass conversion, 20 mM PHB was yielded from 50 mM sodium acetate with a molar conversion efficiency of carbon of 80.0 % and a light-energy conversion efficiency of 3.04 %, which are much higher than the efficiencies of similar in vitro PHB synthesis biosystems. This suggests the promise of installing TMs as a green engine to drive more enzyme cascades.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202111054-sup-0001-misc_information.pdf2 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. U. Bowie, S. Sherkhanov, T. P. Korman, M. A. Valliere, P. H. Opgenorth, H. Liu, Trends Biotechnol. 2020, 38, 766–778;
- 1bK. Petroll, D. Kopp, A. Care, P. L. Bergquist, A. Sunna, Biotechnol. Adv. 2019, 37, 91–108;
- 1cY. H. Zhang, Biotechnol. Adv. 2015, 33, 1467–1483;
- 1dJ. M. Sperl, V. Sieber, ACS Catal. 2018, 8, 2385–2396.
- 2
- 2aT. P. Korman, P. H. Opgenorth, J. U. Bowie, Nat. Commun. 2017, 8, 15526;
- 2bM. A. Valliere, T. P. Korman, N. B. Woodall, G. A. Khitrov, R. E. Taylor, D. Baker, J. U. Bowie, Nat. Commun. 2019, 10, 565.
- 3
- 3aE.-J. Kim, J.-E. Kim, Y.-H. P. J. Zhang, Energy Environ. Sci. 2018, 11, 2064–2072;
- 3bJ. A. Rollin, J. Martin del Campo, S. Myung, F. Sun, C. You, A. Bakovic, R. Castro, S. K. Chandrayan, C. H. Wu, M. W. Adams, R. S. Senger, Y. H. Zhang, Proc. Natl. Acad. Sci. USA 2015, 112, 4964–4969.
- 4C. You, H. Chen, S. Myung, N. Sathitsuksanoh, H. Ma, X.-Z. Zhang, J. Li, Y.-H. P. Zhang, Proc. Natl. Acad. Sci. USA 2013, 110, 7182–7187.
- 5
- 5aH. Taniguchi, K. Okano, K. Honda, Synth. Syst. Biotechnol. 2017, 2, 65–74;
- 5bT. Shi, P. Han, C. You, Y. P. J. Zhang, Synth. Syst. Biotechnol. 2018, 3, 186–195;
- 5cQ. M. Dudley, A. S. Karim, M. C. Jewett, Biotechnol. J. 2015, 10, 69–82.
- 6
- 6aT. Shi, S. Liu, Y. P. J. Zhang, Metab. Eng. 2019, 55, 152–160;
- 6bH. Chen, Y. P. J. Zhang, Crit. Rev. Biotechnol. 2021, 41, 16–33;
- 6cK. Honda, N. Hara, M. Cheng, A. Nakamura, K. Mandai, K. Okano, H. Ohtake, Metab. Eng. 2016, 35, 114–120.
- 7
- 7aD. Yang, Y. Zhang, S. Zhang, Y. Cheng, Y. Wu, Z. Cai, X. Wang, J. Shi, Z. Jiang, ACS Catal. 2019, 9, 11492–11501;
- 7bC. J. Seel, T. Gulder, ChemBioChem 2019, 20, 1871–1897;
- 7cS. H. Lee, D. S. Choi, S. K. Kuk, C. B. Park, Angew. Chem. Int. Ed. 2018, 57, 7958–7985; Angew. Chem. 2018, 130, 8086–8116.
- 8
- 8aK. Y. Lee, S. J. Park, K. A. Lee, S. H. Kim, H. Kim, Y. Meroz, L. Mahadevan, K. H. Jung, T. K. Ahn, K. K. Parker, K. Shin, Nat. Biotechnol. 2018, 36, 530–535;
- 8bS. Berhanu, T. Ueda, Y. Kuruma, Nat. Commun. 2019, 10, 1325;
- 8cG. Wang, K. Castiglione, Catalysts 2018, 9, 12;
- 8dH. Lu, W. Yuan, J. Zhou, P. L. Chong, Appl. Biochem. Biotechnol. 2015, 177, 105–117.
- 9
- 9aD. Pankratov, G. Pankratova, L. Gorton, Curr. Opin. Electrochem. 2020, 19, 49–54;
- 9bE. Cevik, B. B. Carbas, M. Senel, H. B. Yildiz, Biosens. Bioelectron. 2018, 113, 25–31;
- 9cM. Rasmussen, A. Shrier, S. D. Minteer, Phys. Chem. Chem. Phys. 2013, 15, 9062–9065;
- 9dG. Pankratova, D. Pankratov, C. Di Bari, A. Goñi-Urtiaga, M. D. Toscano, Q. Chi, M. Pita, L. Gorton, A. L. De Lacey, ACS Appl. Energy Mater. 2018, 1, 319–323;
- 9eH. Hamidi, K. Hasan, S. C. Emek, Y. Dilgin, H. E. Akerlund, P. A. Albertsson, D. Leech, L. Gorton, ChemSusChem 2015, 8, 990–993.
- 10
- 10aT. E. Miller, T. Beneyton, T. Schwander, C. Diehl, M. Girault, R. McLean, T. Chotel, P. Claus, N. S. Cortina, J. C. Baret, T. J. Erb, Science 2020, 368, 649–654;
- 10bY. Wang, B. An, B. Xue, J. Pu, X. Zhang, Y. Huang, Y. Yu, Y. Cao, C. Zhong, Nat. Chem. Biol. 2021, 17, 351–359.
- 11B. Alkotaini, S. Abdellaoui, K. Hasan, M. Grattieri, T. Quah, R. Cai, M. Yuan, S. D. Minteer, ACS Sustainable Chem. Eng. 2018, 6, 4909–4915.
- 12P. H. Opgenorth, T. P. Korman, J. U. Bowie, Nat. Chem. Biol. 2016, 12, 393–395.
- 13E. Noor, H. S. Haraldsdóttir, R. Milo, R. M. T. Fleming, eQuilibrator—Consistent Estimation of Gibbs Energy Using Component Contributions http://equilibrator.weizmann.ac.il/ 2013.
- 14
- 14aS. Kumari, R. Tishel, M. Eisenbach, A. J. Wolfe, J. Bacteriol. 1995, 177, 2878–2886;
- 14bH. Y. Sagong, H. F. Son, S. Y. Choi, S. Y. Lee, K. J. Kim, Trends Biochem. Sci. 2018, 43, 790–805;
- 14cH. Itoh, T. Shiba, J. Bacteriol. 2004, 186, 5178–5181.
- 15
- 15aD. D. Strand, N. Fisher, D. M. Kramer, J. Biol. Chem. 2017, 292, 11850–11860;
- 15bD. Seigneurin-Berny, D. Salvi, J. Joyard, N. Rolland, Current Protocols in Cell Biology, Wiley, Hoboken, 2008, chap. 3, Unit 3 30.
- 16R. J. Porra, Photosynth. Res. 2002, 73, 149–156.
- 17A. M. Timperio, G. M. D'Amici, C. Barta, F. Loreto, L. Zolla, J. Exp. Bot. 2007, 58, 3695–3710.
- 18B. Andersson, E.-M. Aro, Photodamage and D1 protein turnover in photosystem II, Vol. 11, Springer, Dordrecht, 2001.
- 19M. Medina, FEBS J. 2009, 276, 3942–3958.
- 20P. Joliot, G. N. Johnson, Proc. Natl. Acad. Sci. USA 2011, 108, 13317–13322.
- 21S. N. Chohan, L. Copeland, Appl. Environ. Microbiol. 1998, 64, 2859–2863.
- 22
- 22aA. A. Pantazaki, M. G. Tambaka, V. Langlois, P. Guerin, D. A. Kyriakidis, Mol. Cell. Biochem. 2003, 254, 173–183;
- 22bH. Berndt, H. G. Schlegel, Arch. Microbiol. 1975, 103, 21–30.
- 23
- 23aM. Mamedov, H. Hayashi, N. Murata, Biochim. Biophys. Acta Bioenerg. 1993, 1142, 1–5;
- 23bC. Wang, X. L. Ma, Z. Hui, W. Wang, Photosynthetica 2008, 46, 400–409.
- 24M. D. Mamedov, H. Hayashi, H. Wada, P. S. Mohanty, G. C. Papageorgiou, N. Murata, FEBS Lett. 1991, 294, 271–274.
- 25S. Zhang, T. Yasuo, R. W. Lenz, S. Goodwin, Biomacromolecules 2000, 1, 244–251.
- 26F. Li, L. Li, L. Wu, L. Zhang, L. Zhang, C. Yang, C. Liu, J. Power Sources 2020, 449, 227604.
- 27P. Pesaresi, M. Scharfenberg, M. Weigel, I. Granlund, W. P. Schroder, G. Finazzi, F. Rappaport, S. Masiero, A. Furini, P. Jahns, D. Leister, Mol. Plant. 2009, 2, 236–248.
- 28Y. Satoh, K. Tajima, H. Tannai, M. Munekata, J. Biosci. Bioeng. 2003, 95, 335–341.
- 29D. R. Ort, S. S. Merchant, J. Alric, A. Barkan, R. E. Blankenship, R. Bock, R. Croce, M. R. Hanson, J. M. Hibberd, S. P. Long, T. A. Moore, J. Moroney, K. K. Niyogi, M. A. Parry, P. P. Peralta-Yahya, R. C. Prince, K. E. Redding, M. H. Spalding, K. J. van Wijk, W. F. Vermaas, S. von Caemmerer, A. P. Weber, T. O. Yeates, J. S. Yuan, X. G. Zhu, Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536.
- 30
- 30aJ. Kromdijk, K. Głowacka, L. Leonelli, S. T. Gabilly, M. Iwai, K. K. Niyogi, S. P. Long, Science 2016, 354, 857–861;
- 30bF. Li, C. Liu, S. Streckaite, C. Yang, P. Xu, M. J. Llansola-Portoles, C. Ilioaia, A. A. Pascal, R. Croce, B. Robert, J. Biol. Chem. 2021, 296, 100322.
- 31J. Mozejko-Ciesielska, R. Kiewisz, Microbiol. Res. 2016, 192, 271–282.
- 32K. Xu, X. Chen, R. Zheng, Y. Zheng, Front. Bioeng. Biotechnol. 2020, 8, 660.