Visible Light Induced Brønsted Acid Assisted Pd-Catalyzed Alkyl Heck Reaction of Diazo Compounds and N-Tosylhydrazones
Ziyan Zhang
Department of Chemistry and Biochemistry, University of Texas at Dallas, 800 West Campbell Rd, Richardson, TX, 75080 USA
Search for more papers by this authorNikita Kvasovs
Department of Chemistry and Biochemistry, University of Texas at Dallas, 800 West Campbell Rd, Richardson, TX, 75080 USA
Search for more papers by this authorAnastasiia Dubrovina
Department of Chemistry and Biochemistry, University of Texas at Dallas, 800 West Campbell Rd, Richardson, TX, 75080 USA
Search for more papers by this authorCorresponding Author
Prof. Vladimir Gevorgyan
Department of Chemistry and Biochemistry, University of Texas at Dallas, 800 West Campbell Rd, Richardson, TX, 75080 USA
Search for more papers by this authorZiyan Zhang
Department of Chemistry and Biochemistry, University of Texas at Dallas, 800 West Campbell Rd, Richardson, TX, 75080 USA
Search for more papers by this authorNikita Kvasovs
Department of Chemistry and Biochemistry, University of Texas at Dallas, 800 West Campbell Rd, Richardson, TX, 75080 USA
Search for more papers by this authorAnastasiia Dubrovina
Department of Chemistry and Biochemistry, University of Texas at Dallas, 800 West Campbell Rd, Richardson, TX, 75080 USA
Search for more papers by this authorCorresponding Author
Prof. Vladimir Gevorgyan
Department of Chemistry and Biochemistry, University of Texas at Dallas, 800 West Campbell Rd, Richardson, TX, 75080 USA
Search for more papers by this authorGraphical Abstract
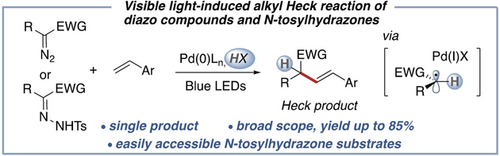
A mild visible light-induced palladium-catalyzed alkyl Heck reaction of diazo compounds and N-tosylhydrazones is reported. This method features Brønsted acid assisted generation of hybrid palladium C(sp3)-centered radical intermediate and broad reaction scope, highlighting the diverse applicability and the potential utility of this protocol in late-stage functionalization.
Abstract
A mild visible light-induced palladium-catalyzed alkyl Heck reaction of diazo compounds and N-tosylhydrazones is reported. A broad range of vinyl arenes and heteroarenes with high functional group tolerance, as well as a range of different diazo compounds, can efficiently undergo this transformation. This method features Brønsted acid-assisted generation of hybrid palladium C(sp3)-centered radical intermediate, which allowed for new selective C−H functionalization protocol.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202110924-sup-0001-misc_information.pdf10.7 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1T. Curtius, Ber. Dtsch. Chem. Ges. 1883, 16, 2230–2231.
10.1002/cber.188301602136 Google Scholar
- 2
- 2aD. M. Hodgson, F. Y. T. M. Pierard, P. A. Stupple, Chem. Soc. Rev. 2001, 30, 50–61;
- 2bH. M. L. Davies, J. R. Manning, Nature 2008, 451, 417–424;
- 2cH. M. L. Davies, J. R. Denton, Chem. Soc. Rev. 2009, 38, 3061–3071;
- 2dH. M. L. Davies, D. Morton, Chem. Soc. Rev. 2011, 40, 1857–1869;
- 2eA. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire, M. A. McKervey, Chem. Rev. 2015, 115, 9981–10080;
- 2fQ.-Q. Cheng, Y. Deng, M. Lankelma, M. P. Doyle, Chem. Soc. Rev. 2017, 46, 5425–5443;
- 2gY. Xia, D. Qiu, J. Wang, Chem. Rev. 2017, 117, 13810–13889;
- 2hŁ. W. Ciszewski, K. Rybicka-Jasińska, D. Gryko, Org. Biomol. Chem. 2019, 17, 432–448;
- 2iD. Zhu, L. Chen, H. Fan, Q. Yao, S. Zhu, Chem. Soc. Rev. 2020, 49, 908–950;
- 2jZ. Yang, M. L. Stivanin, I. D. Jurberg, R. M. Koenigs, Chem. Soc. Rev. 2020, 49, 6833–6847.
- 3
- 3aM. P. Doyle, M. N. Protopopova, Tetrahedron 1998, 54, 7919–7946;
- 3bG. Maas, Chem. Soc. Rev. 2004, 33, 183–190;
- 3cS. E. Reisman, R. R. Nani, S. Levin, Synlett 2011, 2011, 2437–2442;
- 3dL. Liu, J. Zhang, Chem. Soc. Rev. 2016, 45, 506–516;
- 3eS. Chanthamath, S. Iwasa, Acc. Chem. Res. 2016, 49, 2080–2090;
- 3fC. Ebner, E. M. Carreira, Chem. Rev. 2017, 117, 11651–11679.
- 4
- 4aI. D. Jurberg, H. M. L. Davies, Chem. Sci. 2018, 9, 5112–5118;
- 4bZ. Zhang, D. Yadagiri, V. Gevorgyan, Chem. Sci. 2019, 10, 8399–8404.
- 5
- 5aY. Chen, X. P. Zhang, J. Org. Chem. 2004, 69, 2431–2435;
- 5bW. I. Dzik, X. P. Zhang, B. de Bruin, Inorg. Chem. 2011, 50, 9896–9903;
- 5cP. Li, J. Zhao, L. Shi, J. Wang, X. Shi, F. Li, Nat. Commun. 2018, 9, 1972;
- 5dW.-C. C. Lee, D.-S. Wang, C. Zhang, J. Xie, B. Li, X. P. Zhang, Chem 2021, 7, 1588–1601.
- 6
- 6aJ. Jiang, J. Liu, L. Yang, Y. Shao, J. Cheng, X. Bao, X. Wan, Chem. Commun. 2015, 51, 14728–14731;
- 6bM. Giedyk, K. Goliszewska, K. ó Proinsias, D. Gryko, Chem. Commun. 2016, 52, 1389–1392;
- 6cC. te Grotenhuis, N. van den Heuvel, J. I. van der Vlugt, B. de Bruin, Angew. Chem. Int. Ed. 2018, 57, 140–145; Angew. Chem. 2018, 130, 146–151;
- 6dY.-L. Su, G.-X. Liu, J.-W. Liu, L. Tram, H. Qiu, M. P. Doyle, J. Am. Chem. Soc. 2020, 142, 13846–13855;
- 6eJ. Zhao, P. Li, Y. Xu, Y. Shi, F. Li, Org. Lett. 2019, 21, 9386–9390.
- 7
- 7aP. Chuentragool, D. Kurandina, V. Gevorgyan, Angew. Chem. Int. Ed. 2019, 58, 11586–11598; Angew. Chem. 2019, 131, 11808–11812;
- 7bD. Kurandina, P. Chuentragool, V. Gevorgyan, Synthesis 2019, 51, 985–1005;
- 7cW.-J. Zhou, G.-M. Cao, Z.-P. Zhang, D.-G. Yu, Chem. Lett. 2019, 48, 181–191;
- 7dR. Kancherla, K. Muralirajan, A. Sagadevan, M. Rueping, Trends Chem. 2019, 1, 510–523;
- 7eW.-M. Cheng, R. Shang, ACS Catal. 2020, 10, 9170–9196;
- 7fK. P. S. Cheung, D. Kurandina, T. Yata, V. Gevorgyan, J. Am. Chem. Soc. 2020, 142, 9932–9937;
- 7gH.-M. Huang, P. Bellotti, P. M. Pflüger, J. L. Schwarz, B. Heidrich, F. Glorius, J. Am. Chem. Soc. 2020, 142, 10173–10183;
- 7hP. Bellotti, M. Koy, C. Gutheil, S. Heuvel, F. Glorius, Chem. Sci. 2021, 12, 1810–1817.
- 8
- 8aD. Kurandina, M. Parasram, V. Gevorgyan, Angew. Chem. Int. Ed. 2017, 56, 14212–14216; Angew. Chem. 2017, 129, 14400–14404;
- 8bD. Kurandina, M. Rivas, M. Radzhabov, V. Gevorgyan, Org. Lett. 2018, 20, 357–360;
- 8cP. Chuentragool, D. Yadagiri, T. Morita, S. Sarkar, M. Parasram, Y. Wang, V. Gevorgyan, Angew. Chem. Int. Ed. 2019, 58, 1794–1798; Angew. Chem. 2019, 131, 1808–1812.
- 9
- 9aG.-Z. Wang, R. Shang, W.-M. Cheng, Y. Fu, J. Am. Chem. Soc. 2017, 139, 18307–18312;
- 9bG.-Z. Wang, R. Shang, Y. Fu, Org. Lett. 2018, 20, 888–891;
- 9cM. Koy, F. Sandfort, A. Tlahuext-Aca, L. Quach, C. G. Daniliuc, F. Glorius, Chem. Eur. J. 2018, 24, 4552–4555;
- 9dM. Koy, P. Bellotti, F. Katzenburg, C. G. Daniliuc, F. Glorius, Angew. Chem. Int. Ed. 2020, 59, 2375–2379; Angew. Chem. 2020, 132, 2395–2399;
- 9eG. S. Lee, D. Kim, S. H. Hong, Nat. Commun. 2021, 12, 991.
- 10
- 10aN. Kvasovs, V. Iziumchenko, V. Palchykov, V. Gevorgyan, ACS Catal. 2021, 11, 3749–3754;
- 10bW.-M. Cheng, R. Shang, Y. Fu, Nat. Commun. 2018, 9, 5215;
- 10cB. Zhao, R. Shang, G.-Z. Wang, S. Wang, H. Chen, Y. Fu, ACS Catal. 2020, 10, 1334–1343.
- 11See Supporting Information for details.
- 12
- 12aR. F. Heck, Acc. Chem. Res. 1979, 12, 146–151;
- 12bI. P. Beletskaya, A. V. Cheprakov, Chem. Rev. 2000, 100, 3009–3066;
- 12cI. D. Hills, G. C. Fu, J. Am. Chem. Soc. 2004, 126, 13178–13179.
- 13Y. Shao, J. Tong, Y. Zhao, H. Zheng, L. Ma, M. Ma, X. Wan, Org. Biomol. Chem. 2016, 14, 8486–8492.
- 14
- 14aY. Xia, J. Wang, Chem. Soc. Rev. 2017, 46, 2306–2362;
- 14bY. Xia, J. Wang, J. Am. Chem. Soc. 2020, 142, 10592–10605.
- 15W. R. Bamford, T. S. Stevens, J. Chem. Soc. 1952, 4735–4740.
- 16M. Ratushnyy, N. Kvasovs, S. Sarkar, V. Gevorgyan, Angew. Chem. Int. Ed. 2020, 59, 10316–10320; Angew. Chem. 2020, 132, 10402–10406.
- 17
- 17aM. Newcomb, P. H. Toy, Acc. Chem. Res. 2000, 33, 449–455;
- 17bJ. E. Baldwin, Chem. Rev. 2003, 103, 1197–1212.
- 18
- 18aD. A. Petrone, I. Franzoni, J. Ye, J. F. Rodríguez, A. I. Poblador-Bahamonde, M. Lautens, J. Am. Chem. Soc. 2017, 139, 3546–3557;
- 18bY. Hu, Z. Shen, H. Huang, ACS Catal. 2016, 6, 6785–6789;
- 18cR. W. Armbruster, M. M. Morgan, J. L. Schmidt, C. M. Lau, R. M. Riley, D. L. Zabrowsky, H. A. Dieck, Organometallics 1986, 5, 234–237.
- 19
- 19aX. Huang, R. D. Webster, K. Harms, E. Meggers, J. Am. Chem. Soc. 2016, 138, 12636–12642;
- 19bŁ. W. Ciszewski, J. Durka, D. Gryko, Org. Lett. 2019, 21, 7028–7032.