An N-Trifluoromethylation/Cyclization Strategy for Accessing Diverse N-Trifluoromethyl Azoles from Nitriles and 1,3-Dipoles
Ru Zhong Zhang
Jilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis, College of Chemistry, Northeast Normal University, 5268 Renmin Street, Changchun, 130024 China
Search for more papers by this authorRu Xue Zhang
Jilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis, College of Chemistry, Northeast Normal University, 5268 Renmin Street, Changchun, 130024 China
Search for more papers by this authorShuang Wang
Institute of Functional of Material, College of Chemistry, Northeast Normal University, 5268 Renmin Street, Changchun, 130024 China
Search for more papers by this authorCorresponding Author
Dr. Cong Xu
Jilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis, College of Chemistry, Northeast Normal University, 5268 Renmin Street, Changchun, 130024 China
Search for more papers by this authorCorresponding Author
Prof. Wei Guan
Institute of Functional of Material, College of Chemistry, Northeast Normal University, 5268 Renmin Street, Changchun, 130024 China
Search for more papers by this authorCorresponding Author
Prof. Mang Wang
Jilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis, College of Chemistry, Northeast Normal University, 5268 Renmin Street, Changchun, 130024 China
Search for more papers by this authorRu Zhong Zhang
Jilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis, College of Chemistry, Northeast Normal University, 5268 Renmin Street, Changchun, 130024 China
Search for more papers by this authorRu Xue Zhang
Jilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis, College of Chemistry, Northeast Normal University, 5268 Renmin Street, Changchun, 130024 China
Search for more papers by this authorShuang Wang
Institute of Functional of Material, College of Chemistry, Northeast Normal University, 5268 Renmin Street, Changchun, 130024 China
Search for more papers by this authorCorresponding Author
Dr. Cong Xu
Jilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis, College of Chemistry, Northeast Normal University, 5268 Renmin Street, Changchun, 130024 China
Search for more papers by this authorCorresponding Author
Prof. Wei Guan
Institute of Functional of Material, College of Chemistry, Northeast Normal University, 5268 Renmin Street, Changchun, 130024 China
Search for more papers by this authorCorresponding Author
Prof. Mang Wang
Jilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis, College of Chemistry, Northeast Normal University, 5268 Renmin Street, Changchun, 130024 China
Search for more papers by this authorGraphical Abstract
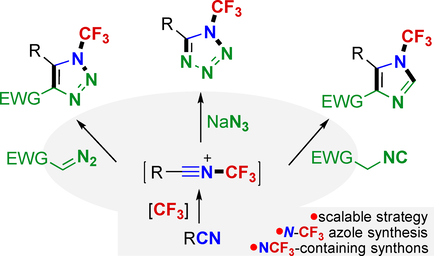
A scalable N-trifluoromethylative cyclization strategy is reported to construct N-CF3 tetrazoles/imidazoles/1,2,3-triazoles. N-CF3 nitrilium derivatives are obtained via the N-trifluoromethylation of nitriles and well used as NCF3-containing synthons in 1,3-dipolar cyclizations for the first time. A generic platform for accessing diverse N-CF3 azoles is thus provided.
Abstract
N-Trifluoromethyl azoles are valuable targets in medicinal chemistry, but their synthesis is challenging. Classical preparation of N-CF3 azoles relies on the functional group interconversions but suffers from tedious N-pre-functionalization and unfriendly agents. Introduction of the CF3 onto the nitrogen of heterocycles provides a direct route to such motifs, but the N-trifluoromethylation remains underdeveloped. Reported here is an alternative and scalable cyclization strategy based on NCF3-containing synthons for constructing N-CF3 azoles. The approach involves the N-trifluoromethylation of nitriles followed by a [3+2] cyclization between resulting N-CF3 nitrilium derivatives and 1,3-dipoles. PhICF3Cl was an effective CF3 source for the transformation. As a result, a generic platform is established to divergently synthesize N-trifluoromethylated tetrazoles, imidazoles, and 1,2,3-triazoles by using sodium azide, activated methylene isocyanides, and diazo compounds as dipoles.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202110749-sup-0001-cif.zip121.4 KB | Supporting Information |
| anie202110749-sup-0001-misc_information.pdf17.9 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see:
- 1a Modern Heterocyclic Chemistry (Eds.: J. Alvarez-Builla, J. J. Vaquero, J. Barluenga), Wiley-VCH, Weinheim, 2011;
- 1bY. Lee, E. Puumala, N. Robbins, L. E. Cowen, Chem. Rev. 2021, 121, 3390–3411;
- 1cH. Gao, J. M. Shreeve, Chem. Rev. 2011, 111, 7377–7436;
- 1dM. E. Easton, H. Choudhary, R. D. Rogers, Chem. Eur. J. 2019, 25, 2127–2140;
- 1eA. Armstrong, J. C. Collins, Angew. Chem. Int. Ed. 2010, 49, 2282–2285; Angew. Chem. 2010, 122, 2332–2335.
- 2
- 2aB. E. Smart, J. Fluorine Chem. 2001, 109, 3–11;
- 2bT. M. Klapötke, Fluorine Chemistry at the Millenium: Fascinated by Fluorine (Eds.: R. E. Banks), Elsevier, Oxford, 2000.
- 3S. Schiesser, H. Chepliaka, J. Kollback, T. Quennesson, W. Czechtizky, R. J. Cox, J. Med. Chem. 2020, 63, 13076–13089.
- 4T. Milcent, B. Crousse, C. R. Chim. 2018, 21, 771–781.
- 5
- 5aT. Miura, T. Ogoshi, K. Ueyama, D. Motoda, T. Iwayama, K. Suzawa, H. Nagamori, H. Ueno, A. Takahashi, K. Sugimoto, WO2013031922A1, 2013;
- 5bP. Samadder, T. Suchánková, O. Hylse, P. Khirsariya, F. Nikulenkov, S. Drápela, N. Straková, P. Vaňhara, K. Vašíčková, H. Kolářová, L. Binó, M. Bittová, P. Ovesná, P. Kollár, R. Fedr, M. Ešner, J. Jaroš, A. Hampl, L. Krejčí, K. Paruch, K. Souček, Mol. Cancer Ther. 2017, 16, 1831–1842;
- 5cS. R. Schow, R. L. Mackman, C. L. Blum, E. Brooks, A. G. Horsma, A. Joly, S. S. Kerwar, G. Lee, D. Shiffman, M. G. Nelson, X. Wang, M. M. Wick, X. Zhang, R. T. Lum, Med. Chem. Lett. 1997, 7, 2697–2702.
- 6
- 6aE. Klauke, Angew. Chem. Int. Ed. Engl. 1966, 5, 848; Angew. Chem. 1966, 78, 829;
- 6bL. M. Yagupolskii, D. V. Fedyuk, K. I. Petko, V. I. Troitskaya, V. I. Rudyk, V. V. Rudyuk, J. Fluorine Chem. 2000, 106, 181–187;
- 6cG. Bissky, G.-V. Röschenthaler, E. Lork, J. Barten, M. Médebielle, V. Staninets, A. A. Kolomeitsev, J. Fluorine Chem. 2001, 109, 173–181;
- 6dT. M. Sokolenko, K. I. Petko, L. M. Yagupolskii, Chem. Heterocycl. Compd. 2009, 45, 430–435.
- 7
- 7aY. Hagooly, J. Gatenyo, A. Hagooly, S. Rozen, J. Org. Chem. 2009, 74, 8578–8582;
- 7bJ. Yu, J.-H. Lin, J.-C. Xiao, Angew. Chem. Int. Ed. 2017, 56, 16669–16673; Angew. Chem. 2017, 129, 16896–16900;
- 7cT. Scattolin, S. Bouayad-Gervais, F. Schoenebeck, Nature 2019, 573, 102–107;
- 7dS. Bouayad-Gervais, T. Scattolin, F. Schoenebeck, Angew. Chem. Int. Ed. 2020, 59, 11908–11912; Angew. Chem. 2020, 132, 12006–12010.
- 8
- 8aK. Niedermann, N. Früh, R. Senn, B. Czarniecki, R. Verel, A. Togni, Angew. Chem. Int. Ed. 2012, 51, 6511–6515; Angew. Chem. 2012, 124, 6617–6621;
- 8bP. S. Engl, R. Senn, E. Otth, A. Togni, Organometallics 2015, 34, 1384–1395;
- 8cP. S. Engl, C. B. Santiago, C. P. Gordon, W. Liao, J. Am. Chem. Soc. 2017, 139, 13117–13125;
- 8dT. Scattolin, E. Bortolamiol, F. Visentin, S. Palazzolo, I. Caligiuri, T. Perin, V. Canzonieri, N. Demitri, F. Rizzolio, A. Togni, Chem. Eur. J. 2020, 26, 11868–11876.
- 9Z. E. Blastik, S. Voltrová, V. Matoušek, B. Jurásek, D. W. Manley, B. Klepetárová, P. Beier, Angew. Chem. Int. Ed. 2017, 56, 346–349; Angew. Chem. 2017, 129, 352–355.
- 10For selected reviews on nitrilium ions, see:
- 10aA. Hegarty, Acc. Chem. Res. 1980, 13, 448–454;
- 10bS. Kanemasa, Sci. Synth. 2004, 19, 53–66;
- 10cT. van Dijk, J. C. Slootweg, K. Lammertsma, Org. Biomol. Chem. 2017, 15, 10134–10144.
- 11K. Niedermann, N. Frh, E. Vinogradova, M. S. Wiehn, A. Moreno, A. Togni, Angew. Chem. Int. Ed. 2011, 50, 1059–1063; Angew. Chem. 2011, 123, 1091–1095.
- 12O. Sala, H. P. Lüthi, A. Togni, M. Iannuzzi, J. Hutter, J. Comput. Chem. 2015, 36, 785–794.
- 13
- 13aC. Xu, J.-X. Liu, W.-B. Ming, Y.-J. Liu, J. Liu, M. Wang, Q. Liu, Chem. Eur. J. 2013, 19, 9104–9109;
- 13bC. Xu, X. Song, J. Guo, S. Chen, J. Gao, J. Jiang, F. Gao, Y. Li, M. Wang, Org. Lett. 2018, 20, 3933–3937;
- 13cC. Xu, W. Huang, R. Zhang, C. Gao, Y. Li, M. Wang, J. Org. Chem. 2019, 84, 14209–14216;
- 13dJ. Guo, C. Xu, X. Liu, M. Wang, Org. Biomol. Chem. 2019, 17, 2162–2168;
- 13eJ. Guo, C. Xu, L. Wang, W. Huang, M. Wang, Org. Biomol. Chem. 2019, 17, 4593–4599;
- 13fW. Huang, C. Xu, J. Yu, M. Wang, J. Org. Chem. 2021, 86, 1987–1999.
- 14As for the formation of imidoyl azides via nucleophilic attack of azides to activated nitriles and followed by a 5-endo cyclization, please see:
- 14aF. Himo, Z. P. Demko, L. Noodleman, K. B. Sharpless, J. Am. Chem. Soc. 2002, 124, 12210–12216;
- 14bF. Himo, Z. P. Demko, L. Noodleman, K. B. Sharpless, J. Am. Chem. Soc. 2003, 125, 9983–9987;
- 14cV. Tona, B. Maryasin, A. de la Torre, J. Sprachmann, L. Gonzalez, Org. Lett. 2017, 19, 2662–2665.
- 15For selected reviews, see:
- 15aG. Höfle, W. Steglich, H. Vorbrüggen, Angew. Chem. Int. Ed. Engl. 1978, 17, 569–583; Angew. Chem. 1978, 90, 602–615;
- 15bE. F. V. Scriven, Chem. Soc. Rev. 1983, 12, 129–162;
- 15cU. Ragnarsson, L. Grehn, Acc. Chem. Res. 1998, 31, 494–501.
- 16
- 16aT. van Dijk, S. Burck, M. K. Rong, A. J. Rosenthal, M. Nieger, J. C. Slootweg, K. Lammertsma, Angew. Chem. Int. Ed. 2014, 53, 9068–9071; Angew. Chem. 2014, 126, 9214–9217;
- 16bT. van Dijk, M. S. Bakker, F. Holtrop, M. Nieger, J. C. Slootweg, K. Lammertsma, Org. Lett. 2015, 17, 1461–1464.
- 17For selected reviews, see:
- 17aR. V. A. Orru, V. G. Nenajdenk in Isocyanide Chemistry, Wiley-VCH, Weinheim, 2012, pp. 109–158;
10.1002/9783527652532.ch4 Google Scholar
- 17bA. V. Lygin, A. de Meijere, Angew. Chem. Int. Ed. 2010, 49, 9094–9124; Angew. Chem. 2010, 122, 9280–9311.
- 18For crystal data for 5 a and 7 a, please see the Supporting Information. Deposition Numbers 2048689 (5 a) and 2092696 (7 a) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 19For recent reviews on diazo compounds, see:
- 19aK. A. Mix, M. R. Aronoff, R. T. Raines, ACS Chem. Biol. 2016, 11, 3233–3244;
- 19bA. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire, M. A. McKervey, Chem. Rev. 2015, 115, 9981–10080.
- 20For recent applications of N-fluoroalkyl 1,2,3-triazoles, see:
- 20aO. Bakhanovich, V. Khutorianskyi, V. Motornov, P. Beier, Beilstein J. Org. Chem. 2021, 17, 504–510;
- 20bD. Tichý, V. Koštál, V. Motornov, I. Klimánková, P. Beier, J. Org. Chem. 2020, 85, 11482–11489;
- 20cV. Motornov, A. Markos, P. Beier, Chem. Commun. 2018, 54, 3258–3261.
- 21
- 21aA. Herschhorn, L. Lerman, M. Weitman, I. O. Gleenberg, A. Nudelman, A. Hizi, J. Med. Chem. 2007, 50, 2370–2384;
- 21bT. Evans, B. Cook, S. Chen, WO 2020205989 A1 20201008, 2020.