Probing Redox Non-Innocence in Iron–Carbene Complexes {Fe=C(H)Ar}10–11 by 1,2H and 13C Pulse Electron Paramagnetic Resonance
Graphical Abstract
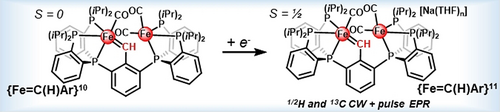
Presented herein are detailed 1/2H and 13C pulse EPR and DFT investigations of an S=1/2 terminal iron–carbene species. These studies reveal substantial spin density localized at the carbene-bound Fe center, in contrast with what has been reported to date for radical metal–carbenoid species. The {Fe=C(H)Ar}11 complex serves as a well-defined reference compound for comparison to data reported for in situ trapped intermediates.
Abstract
We report the synthesis and spectroscopic characterization of a series of iron-carbene complexes in redox states {Fe=C(H)Ar}10–11. Pulse EPR studies of the 1,2H and 13C isotopologues of {Fe=C(H)Ar}11 reveal the high covalency of the Fe–carbene bonding, leading to a more even spin distribution than commonly observed for reduced Fischer carbenes.