Divergent and Modular Synthesis of Terpenoid Scaffolds via a AuI Catalyzed One-Pot Cascade
Huy Tran
Department of Chemistry and Biomolecular Sciences, Centre for Catalysis, Research and Innovation, University of Ottawa, 10 Marie Curie, Ottawa, Ontario, K1N6N5 Canada
Search for more papers by this authorDr. Guillaume Revol
OmegaChem, 480 Rue Perreault, Saint-Romuald, Quebec, G6W7V6 Canada
Search for more papers by this authorAlyson Poyser
Transport Canada, 330 Sparks St., Ottawa, ON, K1A0N8 Canada
Search for more papers by this authorCorresponding Author
Prof. Dr. Louis Barriault
Department of Chemistry and Biomolecular Sciences, Centre for Catalysis, Research and Innovation, University of Ottawa, 10 Marie Curie, Ottawa, Ontario, K1N6N5 Canada
Search for more papers by this authorHuy Tran
Department of Chemistry and Biomolecular Sciences, Centre for Catalysis, Research and Innovation, University of Ottawa, 10 Marie Curie, Ottawa, Ontario, K1N6N5 Canada
Search for more papers by this authorDr. Guillaume Revol
OmegaChem, 480 Rue Perreault, Saint-Romuald, Quebec, G6W7V6 Canada
Search for more papers by this authorAlyson Poyser
Transport Canada, 330 Sparks St., Ottawa, ON, K1A0N8 Canada
Search for more papers by this authorCorresponding Author
Prof. Dr. Louis Barriault
Department of Chemistry and Biomolecular Sciences, Centre for Catalysis, Research and Innovation, University of Ottawa, 10 Marie Curie, Ottawa, Ontario, K1N6N5 Canada
Search for more papers by this authorGraphical Abstract
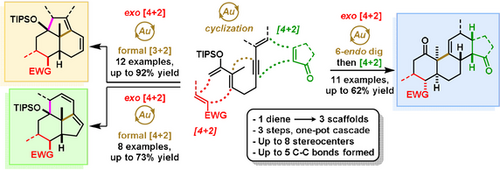
A one-pot sequence involving an exo-Diels–Alder reaction followed by a Lewis acid gold(I) catalyzed cyclization has allowed access to three complex polycyclic scaffolds. Structural diversity is obtained by varying solvent and the ligand of the gold(I) catalyst. An additional Diels–Alder step generates tetracyclic cores possessing up to 5 stereocenters.
Abstract
A one-pot cascade sequence to generate synthetically challenging polycyclic scaffolds is reported utilizing a novel Lewis acid gold catalyst for the key cyclization step, enabling the divergent synthesis of both 6,6,5-tricyclic and 6,6,6,5-tetracyclic cores through both ligand and reaction condition control. We have combined the intrinsic complexity and stereoselectivity of cycloadditions with the electronic and steric properties of gold complexes to selectively generate complex polycyclic scaffolds in a single operation.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202110575-sup-0001-misc_information.pdf18.2 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see:
- 1aB.-C. Hong, A. Raja, V. M. Sheth, Synthesis 2015, 47, 3257–3285;
- 1bL. F. Tietze, G. Brasche, K. M. Gericke, Domino Reactions in Organic Synthesis, Wiley-VCH, 2006;
10.1002/9783527609925 Google Scholar
- 1cJ. Poulin, C. M. Grisé-Bard, L. Barriault, Chem. Soc. Rev. 2009, 38, 3092–3101;
- 1dA. Padwa, Chem. Soc. Rev. 2009, 38, 3072–3081.
- 2For selected recent reviews on gold(I) catalysis, see:
- 2aD. Campeau, D. F. León Rayo, A. Mansour, K. Muratov, F. Gagosz, Chem. Rev. 2021, 121, 8756–8867;
- 2bS. A. Shahzad, M. A. Sajid, Z. A. Khan, D. Canseco-Gonzalez, Synth. Commun. 2017, 47, 735–755;
- 2cA. S. K. Hashmi, in Inventing Reactions (Ed.: L. Gooßen), Springer, Berlin, Heidelberg, 2013, pp. 143–164;
- 2dI. Braun, A. M. Asiri, A. S. K. Hashmi, ACS Catal. 2013, 3, 1902–1907.
- 3
- 3aP. McGee, J. Brousseau, L. Barriault, Isr. J. Chem. 2017, 57, 1–11;
10.1002/ijch.201780101 Google Scholar
- 3bD. Pflästerer, A. S. K. Hashmi, Chem. Soc. Rev. 2016, 45, 1331–1367;
- 3cA. S. K. Hashmi, M. Rudolph, Chem. Soc. Rev. 2008, 37, 1766–1775.
- 4R. Dorel, A. M. Echavarren, Chem. Rev. 2015, 115, 9028–9072.
- 5
- 5aJ. J. Kennedy-Smith, S. T. Staben, F. D. Toste, J. Am. Chem. Soc. 2004, 126, 4526–4527;
- 5bS. T. Staben, J. J. Kennedy-Smith, D. Huang, B. K. Corkey, R. L. LaLonde, F. D. Toste, Angew. Chem. Int. Ed. 2006, 45, 5991–5994; Angew. Chem. 2006, 118, 6137–6140.
- 6F. Barabé, P. Levesque, I. Korobkov, L. Barriault, Org. Lett. 2011, 13, 5580–5583.
- 7
- 7aP. de Frémont, N. Marion, S. P. Nolan, J. Organomet. Chem. 2009, 694, 551–560;
- 7bS. P. Nolan, Acc. Chem. Res. 2011, 44, 91–100.
- 8
- 8aD. J. Gorin, B. D. Sherry, F. D. Toste, Chem. Rev. 2008, 108, 3351–3378;
- 8bC. C. Chintawar, A. K. Yadav, A. Kumar, S. P. Sancheti, N. T. Patil, Chem. Rev. 2021, 121, 8478–8558;
- 8cY. Wei, M. Shi, ACS Catal. 2016, 6, 2515–2524.
- 9
- 9aP. McGee, G. Bétournay, F. Barabé, L. Barriault, Angew. Chem. Int. Ed. 2017, 56, 6280–6283; Angew. Chem. 2017, 129, 6377–6380;
- 9bJ. L. Mascareñas, I. Varela, F. López, Acc. Chem. Res. 2019, 52, 465–479;
- 9cF. Lopez, J. L. Mascareñas, Beilstein J. Org. Chem. 2011, 7, 1075–1094.
- 10C. Nieto-Oberhuber, S. López, A. M. Echavarren, J. Am. Chem. Soc. 2005, 127, 6178–6179.
- 11
- 11aS. Harada, S. Nakashima, S. Sekino, W. Oishi, A. Nishida, Chem. Asian J. 2020, 15, 483–486;
- 11bS. Harada, T. Morikawa, A. Nishida, Org. Lett. 2013, 15, 5314–5317;
- 11cY. Sudo, D. Shirasaki, S. Harada, A. Nishida, J. Am. Chem. Soc. 2008, 130, 12588–12589.
- 12Deposition Numbers 2098879 (for 10aaa), 1967542 (for 10aba), 1967540 (for 10cba), 1967541 (for 10abd), 1967538 (for 10abb), and 1967646 (for 8cf) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 13J.-F. Brazeau, S. Zhang, I. Colomer, B. K. Corkey, F. D. Toste, J. Am. Chem. Soc. 2012, 134, 2742–2749.
- 14
- 14aCrystallographic data for [L5AuNCMe][Cl] shows that the ∢ P-Au-Cl=176.6°. See deposition number 1967543 for crystallographic data. Deposition Number 1967543 contains the supplementary crystallographic data. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 14bFor additional examples of bent P-Au-Cl angles, see: E. Herrero-Gómez, C. Nieto-Oberhuber, S. López, J. Benet-Buchholz, A. M. Echavarren, Angew. Chem. Int. Ed. 2006, 45, 5455–5459; Angew. Chem. 2006, 118, 5581–5585.
- 15D. Benitez, E. Tkatchouk, A. Z. Gonzalez, W. A. Goddard, F. D. Toste, Org. Lett. 2009, 11, 4798–4801.
- 16H. Kusama, Y. Karibe, R. Imai, Y. Onizawa, H. Yamabe, N. Iwasawa, Chem. Eur. J. 2011, 17, 4839–4848.