Synthesis of Functionalized Silsesquioxane Nanomaterials by Rhodium-Catalyzed Carbene Insertion into Si−H Bonds
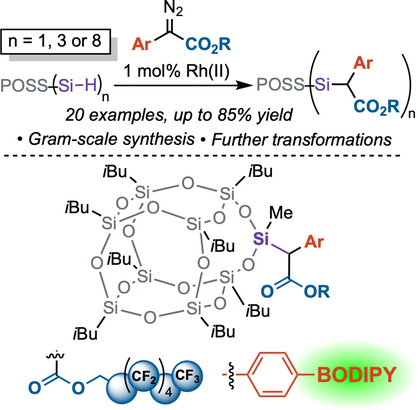
Graphical Abstract
Carbene insertion into Si−H bonds of silsesquioxane silanes was accomplished using RhII catalysis and diazo compounds as carbene precursors. Silsesquioxanes with up to eight Si−H bonds were functionalized up to gram-scale. Diazo compounds with a fluorinated octyl group and BODIPY fluorophore were accessed to highlight the utility of diazo chemistry. Further transformations including a cross-coupling and ester deprotection are demonstrated.
Abstract
We report carbene insertion into Si−H bonds of polyhedral oligomeric silsesquioxanes (POSS) for the synthesis of highly functionalized siloxane nanomaterials. Dirhodium(II) carboxylates catalyze insertion of aryl-diazoacetates as carbene precursors to afford POSS structures containing both ester and aryl groups as orthogonal functional handles for further derivatization of POSS materials. Four diverse and structurally varied silsesquioxane core scaffolds with one, three, or eight Si−H bonds were evaluated with diazo reactants to produce a total of 20 new POSS compounds. Novel diazo compounds containing a fluorinated octyl group and boron-dipyrromethene (BODIPY) chromophore demonstrate the use of highly functionalized substrates. Transformations of aryl(ester)-functionalized POSS compounds derived from this method are demonstrated, including ester hydrolysis and Suzuki–Miyaura cross-coupling.