Tumor-Cell-Specific Targeting of Ibrutinib: Introducing Electrostatic Antibody-Inhibitor Conjugates (AiCs)
Graphical Abstract
The synthesis, in vitro and in vivo evaluation of a vesicular ibrutinib-Cy3.5 hosting nanocarrier is reported. In vivo, it shows a significant enrichment of the drug in xenograft lymphoma tumors in immune-compromised mice and gave a significantly better response at much lower dosage than the original untargeted drug.
Abstract
Ibrutinib is an inhibitor of Bruton's tyrosine kinase that has been approved for the treatment of patients with chronic lymphocytic leukemia, mantle cell lymphoma and Waldenstrom's macroglobulinemia and is connected with toxicities. To minimize its toxicities, we linked ibrutinib to a cell-targeted, internalizing antibody. To this end, we synthesized a poly-anionic derivate, ibrutinib-Cy3.5, that retains full functionality. This anionic inhibitor is complexed by our anti-CD20-protamine targeting conjugate and free protamine, and thereby spontaneously assembles into an electrostatically stabilized vesicular nanocarrier. The complexation led to an accumulation of the drug driven by the CD20 antigen internalization to the intended cells and an amplification of its pharmacological effectivity. In vivo, we observed a significant enrichment of the drug in xenograft lymphoma tumors in immune-compromised mice and a significantly better response to lower doses compared to the original drug.
Introduction
In the last decades, efforts in the analysis of cancer-driving molecular pathways have led to significant progress in the development of specific therapies.1 The emerging techniques, that is, the identification of tumor specific mutations, production of antibodies and screening methods to identify inhibiting agents were combined and tested in a plethora of clinical studies. However, cancer is still a harmful disease and a leading cause of death worldwide.
Usually, antibody-drug-conjugates (ADC) consist of an antibody, a cytotoxic molecule and a linker. The cell-type specific antibody provokes internalisation into the cancer cell, next a rather unspecific but efficient cytotoxic molecule kills the cells as soon as it is internalized.2, 3 The chemical linker between antibody and cytotoxic molecule is either intracellularly cleavable, that is, at lower pH values within the endosomal vesicle, or uncleavable to prevent early liberation of the dangerous cytotoxic molecule.4 This concept has several advantages such as more cell-type specific targeting. However, in the last 20 years less than 10 ADCs were FDA-approved.2, 3 One example is gemtuzumab-ozogamicin (GO, MylotargR), which is composed of the anti-CD33-antibody gemtuzumab that mainly targets myeloid cells that is, in acute myeloid leukemia, linked to the cytotoxic ozogamicin.5 FDA-approved in 2000, GO was withdrawn 2010 due to high side effects and low survival advantages, which possibly was explainable by the leakiness of the cleavable hydrazone linker.4 With modified doses and application, GO was reapproved by the FDA in 2017. Problems with this and other ADCs are: Lack of internalization efficiency of the antibody demands higher doses, lack of specificity of the cytotoxic molecules leads to spill-over and side-effects, and various ways for the cancer cell to acquire resistance, especially when the doses have to be lowered due to toxicity.
Ibrutinib as a small molecule inhibitor significantly improved treatment of patients with mantle cell lymphoma and chronic lymphocytic leukemia.6 Ibrutinib is an orally available covalent inhibitor of the Bruton's tyrosine kinase (BTK) binding at cysteine 481.7 This leads to an irreversible inhibition of the autophosphorylation site and downstream signaling cascade. Since the respective cells are dependent on this signaling, ibrutinib treatment leads to effective growth inhibition and induction of apoptosis, depending on cell-intrinsic determinants.8 However, also the application of untargeted ibrutinib can provoke side effects7 and can lead to acquired resistance mechanisms.9 To combine both targeted therapy agents, we based our approach on an antibody-conjugation strategy that we initially generated to target siRNA via antibodies into a tumor cell.10 Accordingly, we conjugated the anti-CD20 monoclonal antibody (mAB, α) rituximab via the linker sulfo-SMCC to the cationic peptide protamine and simultaneously exploited free protamine. Binding of an anionic partner molecule and formation of a conjugate occurs by electrostatic binding, and we therefore needed an appropriate anionic small molecule partner. Hence, we designed and synthesized an anionic ibrutinib by adding the fluorescent marker Cy3.5TM representing anionic charges in one site of the molecule and offering also a fluorescence readout for analysis.
Here, we present data about the synthesis, binding capacity, specificity, and efficacy of this antibody-inhibitor conjugate in form of an electrostatic nanocarrier in models of diffuse large B-cell lymphoma (DLBCL). DLBCL represents the most frequent lymphoma subtype in adults.11 The introduction of rituximab has made significant impact on the outcome of DLBCL patients. The combination of rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) represents the standard first-line therapy in the vast majority of patients. However, patients who are refractory to first-line treatment or relapsing after initial response are characterized by poor survival,7 indicating that novel therapeutic approaches are urgently needed. Ibrutinib targets B-cell receptor (BCR) signaling that is critical for survival of subsets of DLBCL. As a drawback, the ATP-pocket target cysteine residue is conserved among nine other tyrosine kinases.12, 13 These processes lead to higher dosage of ibrutinib, its interception by irrelevant cells and in addition even to adverse effects, which could be partly due to the bystander effects on targets other than BTK.14 Next, prolonged ibrutinib dosage can lead to development of resistance.9
Results and Discussion
To circumvent some of the disadvantages discussed above, we intended to construct a conjugate that consists of a tumor-targeting antibody and an inhibitor of a tumor-driving kinase, which confers double specificity and therefore safer application, and which assembles only by electrostatic interaction. As an example, we chose the B-cell specific anti-CD20 antibody rituximab15 and the B-cell pathway inhibitor ibrutinib,14, 16 mainly because both are part of standard therapies.
First, we converted the uncharged ibrutinib to the strongly anionic compound ibrutinib-Cy3.5, which allowed to bind by means of electrostatic force to our cationic protamine-based carrier system to form an antibody-inhibitor-complex (Scheme 1 A). The cyanine dye Cy3.5 exhibits strong anionic character by exposing four sulfonic acid groups as potential electrostatic binders. It is important to concentrate the anionic charges on one site of the molecule and to retain an overall linear shape to form the nanocarrier. In addition, the cyanine dye allows a fluorescence read out in all stages of evaluation. According to the literature,17 we synthesized a protected amino-functionalized ibrutinib-derivative 9 starting with the commercially available pyrazolopyrimidine which was subsequently iodinated and substituted with 4-phenyloxy-benzene boronic acid via Suzuki-coupling to form the main part 2 of the ibrutinib core structure. To receive high binding affinity (S)-N-Boc-3-hydroxypiperidine was installed via Mitsunobu reaction forming compound 4. After deprotection of the piperidine moiety an α,β-unsaturated linker 7 (Michael acceptor) was introduced for irreversible binding to the target.18 The resulting Boc-protected amine 9 represents the lead structure for labelling with the cyanine dye Cy3.5 yielding ibrutinib-Cy3.5 (Scheme 1 A). Ibrutinib-Cy3.5 was then incorporated into the electrostatic nanocarrier (Scheme 1 B).
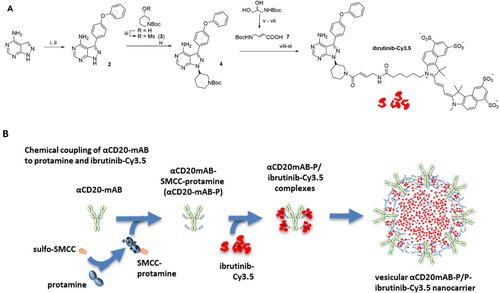
Synthesis of ibrutinib-Cy3.5 and the vesicular αCD20-mAB-P/free P-ibrutinib-Cy3.5 nanocarrier. A: Synthesis of ibrutinib-Cy3.5: (i) NIS, DMF, 80 °C; (ii) p-phenoxyphenylboronic acid, Pd(PPh3)4, KOH, dioxane/water 5:1, MW, 180 °C, 10 min, 58 % (two steps); (iii) MeSO2Cl, Et3N, DCM, rt, 77 %; (iv) K2CO3, DMF, 80 °C, overnight, 46 %; (v) NaIO4, H2O, rt, 84 %; (vi) (EtO)2P=OCH2COOEt, NaH, THF, 0 °C to rt, 35 %; (vii) LiOH, THF/H2O 2:1, rt, 83 %; (viii) 4 M HCl(g)/dioxane, EtOAc/MeOH 1:1, rt, 98 %; (ix) 7, PyAOP, DIPEA, MeCN, 72 %; (x) 4 M HCl(g)/dioxane, EtOAc/MeOH 1:1, rt; (xi) sulfo-Cy3.5 NHS ester, DIPEA, DMF, rt, 80 %, two steps. B: Schematic overview: the αCD20-mAB was conjugated to SMCC-protamine via cysteine sulfhydryls (SMCC) to obtain αCD20-mAB-protamine (αCD20-mAB-P) and then in the presence of additional free protamine anionic ibrutinib-Cy3.5 electrostatically binds to the cationic protamines forming the vesicular αCD20-mAB-P/free P-ibrutinib-Cy3.5 nanocarrier.
Besides the strong polyanionic character of the Cy3.5 dye, the conjugate had the advantage of being easily traceable in vitro and in vivo in form of a red fluorescence (Figure 1).
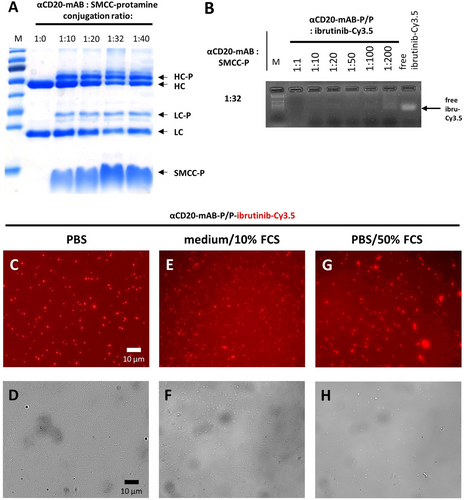
Properties of the αCD20-mAB-protamine-ibrutinib-Cy3.5/free P nanocarrier. A: SDS-PAGE illustrating molecular weight shifts by protamine conjugation of heavy chain (HC to HC-P) and light chain (LC to LC-P) of αCD20-mAB conjugated to rising amounts of SMCC-protamine. B: electromobility shift assays showing the electrostatic loading capacity of ibrutinib-Cy3.5 to conjugates from A. The conjugation ratio of 1:32 was optimal in terms of loading capacity of more than 100 mol ibrutinib-Cy3.5 per mol of αCD20-mAB-protamine. C–H: Stability after 1 h-auto-assembly of αCD20-mAB-protamine, free protamine and ibrutinib-Cy3.5 in a 1:20 ratio and subsequent incubation for 24 h in PBS (C, D), and in challenging conditions such as cell culture medium RPMI/10 % FCS (E, F) and PBS/50 % FCS (G, H). C, E, G, Cy3.5 fluorescence, D, F, H, phase contrast. α, anti; FCS, fetal calf serum.
For the formation of the carrier monoclonal antibody, αCD20-mAB was conjugated to SMCC-protamine by cysteines of the IgG backbone (Scheme 1 B). The conjugation was observable by molecular weight shifts in the IgG heavy chain as well as the light chain, indicating the binding of one protamine peptide per light and heavy chain of the IgG (Figure 1 A). The resulting αCD20-mAB-protamine conjugate was successfully tested for CD20-receptor binding and internalization by flow cytometry analysis (Support. Figure 2 A). To form a carrier conjugate suitable to complex ibrutinib-Cy3.5 efficiently, a certain molar excess of free non-bound SMCC-protamine over the carrier αCD20-mAB-P is necessary, here, a 32:1 molar ratio was shown to be optimal in terms of stable complexation of ibrutinib-Cy3.5 (Figure 1 B, Support. Figure 2 B). In these assays, αCD20-mAB-protamine/free protamine (αCD20-mAB-P/P) complex allowed to bind more than 100 mol ibrutinib-Cy3.5 per mol of carrier antibody by means of electrostatic force to our protamine-based carrier system. This carrier assembly depends on the presence of an excess of free (SMCC-)protamine, as depletion of free SMCC-protamine leads to non-assembly or destruction of the carrier (Support. Figure 3). We therefore conclude that free protamine is essential for nanocarrier formation, which we now call αCD20-mAB-P/P-ibrutinib-Cy3.5 to indicate this composition.
The building of an antibody-inhibitor-complex in form of stable nanoparticles could be detected in fluorescence microscopy (Figure 1 C–H), which are stable in serum (Figure 1 E–H) under conditions as published for other nanoparticles.19 Importantly, as ibrutinib-Cy3.5 is detectable by fluorescence, this brings along excellent tracing abilities for all downstream applications.
When incubated in vitro, αCD20-mAB-P/P loaded with ibrutinib-Cy3.5 led to the assembly of electrostatically stabilized nanoparticles exposing red Cy3.5 fluorescence (Figure 2). In fluorescence microscopy, first regular shaped vesicular structures (Figure 1 C,D), later irregular shaped aggregates larger than 2 μm plus smaller particles were detected, this process was not seen, if unmodified αCD20-mAB was used to complex ibrutinib-Cy3.5, or modified αCD20-mAB-P/free protamine was used to complex hydrophobic ibrutinib (ImbruvicaR) (not shown). The electrostatic particles seen in light microscopy (Figure 2 A,B) were also validated in electron microscopy (Figure 2 C), where a multitude of smaller particles ranging <100–200 nm were detected (Figure 2 C), which induced us to choose the term “nano”carrier.
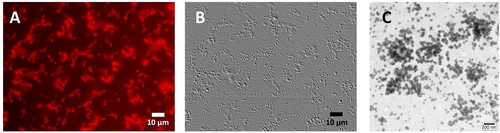
Electrostatic nanoparticle formation by αCD20-mAB-protamine/free protamine-ibrutinib-Cy3.5. The carrier antibody-protamine conjugate was loaded with anionic ibrutinib-Cy3.5 in 1:20 ratio and applied to cell-culture treated glass slides for fluorescence microscopy (A, B) or copper grids for phospho-Wolfram negative stained electron microscopy (C). Here, the electrostatic loading led to the formation of numerous aggregates, where the larger aggregates showed intense Cy3.5 fluorescence (A) and were visible in light microscopy using emboss dynamic filter to illustrate 3D structures through contrast enhancement (B). In transmission electron microscopy (C), negative staining led to roughly the same range of particle sizes but revealed the presence of a plethora of smaller vesicles (C) undetectable in light microscopy. α, anti.
Next, we investigated the efficacy of this αCD20-mAB-P/P-ibrutinib-Cy3.5 nanocarrier in different cellular model systems.
First, we examined the internalisation into CD20-positive DLBCL cells via Cy3.5 fluorescence. HBL1 and TMD8 lymphoma cells treated overnight with uncoupled ibrutinib-Cy3.5 show decent red fluorescence marking of cells (Figure 3 E and Support. Figure 4 E), which was intensified, when ibrutinib-Cy3.5 was complexed and transported with αCD20-mAB-P/P (Figure 3 F and Support. Figure 4 F). This indicated a beneficial process of internalization by the CD20 receptor over the untargeted uptake mechanisms for ibrutinib-Cy3.5 anion without carrier antibody implementation (Figure 3 E compared to 3 F). Next, a 72 hrs treatment of cells with the conjugates show a singular band of covalent Cy3.5 marking of a 70 kDa protein in an SDS PAGE electrophoresis, indicating binding and functionality of the modified ibrutinib-Cy3.5 compound (Figure 3 G; see Support. Figure 4 G and H for 24 h and 48 h treatment). For fluorescence detection of BTK, the gel had to be considerably overloaded, in order to show equal loading of lanes and identification of BTK, so next we blotted the gel for immunodetection of BTK after fluorescence detection. Indeed, a band representing BTK appeared at the same position as seen in the Cy3.5 fluorescence, indicating that ibrutinib-Cy3.5 had covalently bound exclusively to BTK, as anticipated (Figure 3 G; see Support. Figure 4 G,H).
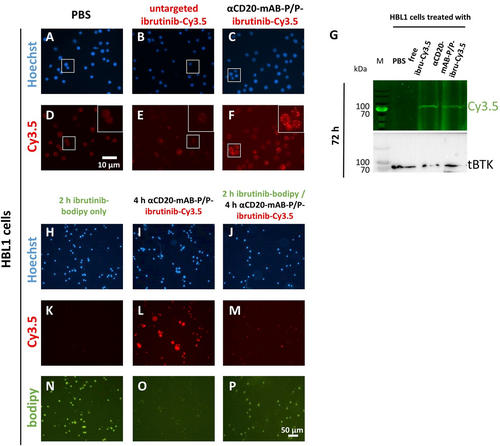
Cellular targeting of Bruton's kinase BTK by αCD20-mAB-P/P-complexed ibrutinib-Cy3.5. A–F: fluorescence microscopy of HBL1 DLBCL cells treated with targeting conjugates and controls showing a marked intracellular enrichment of Cy3.5-signals. G: lysates from cell treated for 72 h with targeting conjugates and controls were subjected to SDS PAGE and illuminated for Cy3.5 signals. Here a clear band of 70 kDa, identified as BTK by parallel immunoblot, was covalently marked by ibrutinib-Cy3.5, indicating binding and thus functionality of the ibrutinib-Cy3.5 derivate. H–P: fluorescence microscopy of HBL1 DLBCL cells pre-treated with ibrutinib-bodipy (green, N and P) do not show intracellular enrichment of Cy3.5-signals after αCD20-mAB-P/P-ibrutinib-Cy3.5 treatment (M, compared to L). α, anti.
Moreover, HBL1 cells were incubated with ibrutinib-bodipy for 2 h, washed and treated with αCD20-mAB-P/P-ibrutinib-Cy3.5. Cells incorporate ibrutinib-bodipy (Figure 3 N,P), but Cy3.5 fluorescence only appears in non-pretreated cells (Figure 3 L) and not in cells pre-treated with ibrutinib-bodipy (Figure 3 P). Some subcellular red vesicles indicate CD20-mediated internalization of ibrutinib-Cy3.5 (Figure 3 P), but a pattern that hints at BTK binding (see Figure 3 L for ibrutinib-Cy3.5 and Figure 3 N,P for ibrutinib-bodipy) does not occur. This is also true after 24 h of αCD20-mAB-P/P-ibrutinib-Cy3.5 treatment (Support. Figure 5) and after pre-incubation with and washout of non-fluorescent ibrutinib (Support. Figure 6).
The functional effect of covalent targeting of BTK by ibrutinib is the inhibition of BTK autophosphorylation ability.14 Therefore, we analysed the phosphorylation status of BTK in DLBCL cells after ibrutinib-Cy3.5 treatment with and without complexation in the nanocarrier (Figure 4 A and Support. Figure 7 A). Cells were treated for 72 hrs with PBS, uncomplexed ibrutinib-Cy3.5 and with the αCD20-mAB-P/P-ibrutinib-Cy3.5 complex, lysed and subjected to Western blot analysis. We found that phosphorylation of BTK at tyrosine 223, detected by a specific phospho-BTK-antibody was significantly decreased in HBL1 (Figure 4 A, left panel) and TMD8 cells (Support. Figure 7 A, right panel) upon treatment with ibrutinib-Cy3.5, irrespective if it was complexed or not. This was in accordance with its binding to BTK as depicted in Figure 3 G. Expression of total BTK was mildly influenced (Figure 4 A and Support. Figure 7 A). We concluded that the synthesized ibrutinib-Cy3.5 conjugate retains full functionality in terms of binding the target molecule BTK as well as inactivation of BTK autophosphorylation.
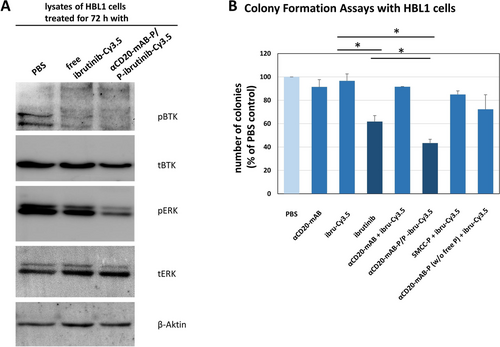
Physiological and functional consequences of BTK-inactivation by αCD20-mAB-protamine/free protamine-ibrutinib-Cy3.5 treatment in DLBCL cell lines. A: HBL1 cells were treated by the respective conjugates shown for 72 hrs, lysed and subjected to SDS-PAGE and Western blotting for phospho-BTK (pBTK), total BTK (tBTK), phospho-ERK (p-ERK), total-ERK (t-ERK) and actin as a loading control. Here, untargeted ibrutinib-Cy3.5 inhibited the phosphorylation of BTK a bit less than αCD20-mAB-P/P-ibrutinib-Cy3.5, the difference of expected downstream phosphorylation targets such as ERK was more pronounced: Here, only αCD20-mAB-P/P-ibrutinib-Cy3.5 treatment was able to reduce ERK phosphorylation. B: In colony formation assays, untargeted ibrutinib-Cy3.5 modestly reduced colony growth of HBL1 cells, while the specific targeting of ibrutinib-Cy3.5 by αCD20-mAB-P/P boosted the colony growth reduction to below 50 %. In order to demonstrate the significance of the free protamine in the conjugate construct, we depleted it from the conjugate mixture, the application of this combination revealed no more colony forming reduction than the single application of ibrutinib-Cy3.5, so the antibody conjugate has lost its targeting ability (B, rightmost bar). α, anti.
Interestingly, in all tested lymphoma cell lines, the lymphoma-specific αCD20-mAB-P/P-ibru-Cy3.5 nanocarrier system significantly inhibited colony growth in soft agar cultures. This was observed to a much lower degree for ibrutinib or ibrutinib-Cy3.5 as single agents, and not if unmodified rituximab (αCD20-mAB) was used (HBL1: Figure 4 B, and TMD8: Support. Figure 7 B). This colony-assay is used for quantification of anchorage-independent clonal cell growth and is a standard in vitro surrogate for tumorigenicity in vivo. We therefore argue that a robust therapeutic effect of ibrutinib-Cy3.5 is only seen, when the anionic compound is assembled into a stable electrostatic nanoparticle composed of the cationic αCD20-mAB-protamine/free protamine carrier complex and the anionic cargo effector.
Next, we explored the functional consequences of BTK inactivation by αCD20-mAB-P/P-ibrutinib-Cy3.5 on DLBCL cell lines in terms of induction of apoptosis. Here, in HBL1 (Figure 5) as well as in TMD8 cells (Support. Figure 8), αCD20-mAB-P/P-ibrutinib-Cy3.5 treatment offered superior induction of apoptosis signals (Figure 5 and Support. Figure 8, rightmost bars, respectively), whereas the uncomplexed ibrutinib-Cy3.5 treatment showed only mild effects in comparison to the targeted treatment as well as the free ibrutinib treatment. We therefore argue that the targeted treatment of αCD20-mAB-P/P-ibrutinib-Cy3.5 leads to an accumulation of active ibrutinib-Cy3.5 in the cells and hence to a more severe induction of apoptosis than the uncomplexed ibrutinib-Cy3.5. It also seems that the anionic molecule ibrutinib-Cy3.5, if uncomplexed is less accessible or at least less effective to the cells as the hydrophobic free ibrutinib, judged by the lower induction of apoptosis as compared to free ibrutinib.
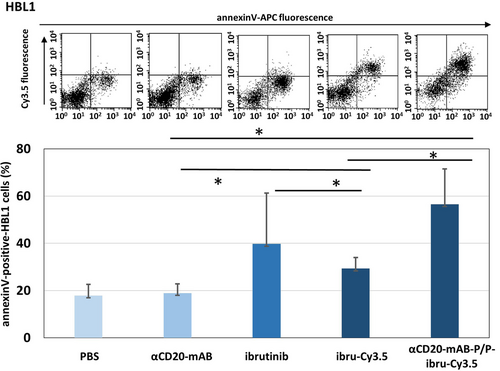
Induction of apoptosis of BTK targeting by αCD20-mAB-P/P complexed ibrutinib-Cy3.5 treatment in the DLBCL cell line HBL1. HBL1 cells were treated by the respective conjugates shown for 72 hrs and subjected to AnnexinV-staining. Apoptotic cells were detected by AnnexinV-expression (upper panel, X-Axis) by flow cytometry, while increased internalized ibrutinib-Cy3.5 fluorescence is seen by fluorescence in Y-axis (upper panel) especially in the αCD20-mAB-P/P complexed ibrutinib-Cy3.5 treated cells. Values from upper right and lower right gates were counted. Lower panel: AnnexinV-positive cells in three independent experiments were summarized. P<0.05, 2-sided T-test. α, anti.
For an in vivo application, ibrutinib-Cy3.5 had an additional advantage over free ibrutinib: In contrast to the hydrophobic drug, it is polar and water-soluble and thus systemically applicable. Thus, we turned to an in vivo treatment of a human DLBCL-xenograft model in mice by intra-peritoneal (i.p.) application of the drug conjugates using two different dosages of the conjugates, 4 mg kg−1 and 8 mg kg−1, calculated for the antibody moiety, which implicates the targeted delivery of only 15 to 30 nanomol of ibrutinib-Cy3.5 per single dose, twice a week.
Even at these low doses, αCD20-mAB-P/P-ibrutinib-Cy3.5 significantly reduced lymphoma growth to below 20 % of those of the controls in in vivo NOD-Scid gamma (NSG) mouse xenografts of HBL1 lymphomas (see Figure 6). Because the majority of the control animals had to be sacrificed due to excessive tumor growth, the tumor growth curve had to be discontinued and then was converted to a survival curve (see Kaplan-Meyer plot in Figure 6 B). While the control groups, that is, the ibrutinib-Cy3.5 monotherapy, the carrier antibody as single therapy, and PBS had to be terminated on day 9 and 16, respectively, the group that received the unmodified ibrutinib (15 or 30 nmol per single dose, intra-peritoneal application) survived until day 22 (Figure 6 B). In contrast, the i.p. treatment with 4 mg kg−1 αCD20-mAB-P/P-ibrutinib-Cy3.5 led to survival up to 36 days after treatment start (Figure 6 B). In a second experiment (Figure 6 C,D), we used the same xenograft model of HBL1 cells in NSG mice to test the application of 8 mg kg−1 αCD20-mAB-P/P-ibrutinib-Cy3.5 along with its respective controls. This time, we introduced a special control group into the survey, which was the combination of the un-modified αCD20-mAB combined with the Cy3.5-conjugated ibrutinib derivate. This combination was ineffective to suppress colony growth (Figure 4) and was not able to form electrostatic aggregates (not shown). In this in vivo experiment, the combination of 8 mg kg−1 αCD20-mAB plus ibrutinib-Cy3.5 equally did not slow down tumor growth (violet curves in Figure 6 C, lower panel) and did not show a positive effect on survival (Figure 6 D).
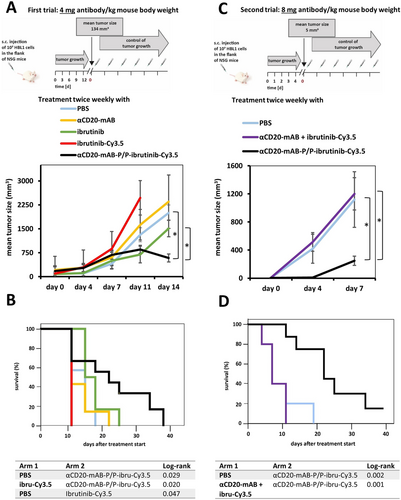
In vivo application of αCD20-mAB-P/P-ibrutinib-Cy3.5 to HBL1 mouse xenografts reduces tumor growth and prolongs survival. A: HBL1 cells were subcutaneously transplanted to NOD-Scid gamma (NSG) immunodeficient mice, tumors developed to a palpable mean size of 134+/- 80 mm3, then mice were randomized to treatment groups and treated with PBS control, 4 mg kg−1 αCD20-mAB, non-modified free ibrutinib (15 nmol (trial A) or 30 nmol (trial B) per single application, i.p.), ibrutinib-Cy3.5 (15 nmol (trial A) or 30 nmol (trial B) per single application, i.p.) and 4 mg kg−1 of αCD20-mAB-P/P-ibrutinib-Cy3.5 conjugate twice a week. Below: Tumors of mice treated with PBS and all the respective monotherapy controls excessively continued to grow to day 14, when the most animals had to be sacrificed because of legal regulations, whereas only the 4 mg kg−1 αCD20-mAB-P/P-ibrutinib-Cy3.5 group decreased in median tumor volume. B: After the termination of the tumor growth curve survey in A, the experiment was continued as a survival curve (Kaplan-Meyer plot). While the ibrutinib-Cy3.5 and the PBS group had to be terminated on days 9 and 16, respectively, the un-modified ibrutinib-treated group (ibrutinib) survived to day 22. Conversely, the αCD20-mAB-P/P-ibrutinib-Cy3.5 treated mice showed a much more decreased tumor growth and consequently the mice survived until day 36. C: Here, we repeated the xenograft model treatment, but starting at day 4 after transplantation at a mean tumor size of 5 mm3 with the administration of the elevated dose of 8 mg kg−1 of the αCD20-mAB-P/P-ibrutinib-Cy3.5, along with the control of un-modified αCD20-mAB plus ibrutinib-Cy3.5 exposing no electrostatic assembly (violet) of the drug. The latter group was ineffective to slow down tumor growth in contrast to the 8 mg kg−1 αCD20-mAB-P/P-ibrutinib-Cy3.5 which reduced tumor growth to below 20 %. D: Likewise, the experiment was continued as a survival curve, where the 8 mg kg−1 αCD20-mAB-P/P-ibrutinib-Cy3.5 (black line) survived up to 40 days post treatment in contrast to controls (between 10 and 20 days). α, anti.
In contrast, the 8 mg kg−1 αCD20-mAB-P/P-ibrutinib-Cy3.5 combination effectively slowed down tumor growth to less than 20 % (Figure 6 C, lower panel) and prolonged survival to more than 40 days compared to 10/20 days (Figure 6 D), when the experiment had to be terminated for reasons of animal welfare.
After sacrificing the mice, organs were prepared and subjected to an in vivo biodistribution study by ex vivo analysis of Cy3.5-dependent fluorescence signals. Here, in contrast to ibrutinib-Cy3.5-monotherapy treated mice (Figure 7 E–G), αCD20-mAB-P/P-ibrutinib-Cy3.5 treated mice showed marked enrichment of Cy3.5-dependent fluorescence signals within the tumor tissue (Figure 7 H–J), but no sequestration of Cy3.5-fluorescence signals to non-tumor tissues (Figure 7 I), all seen by ex vivo imaging of mouse organs.
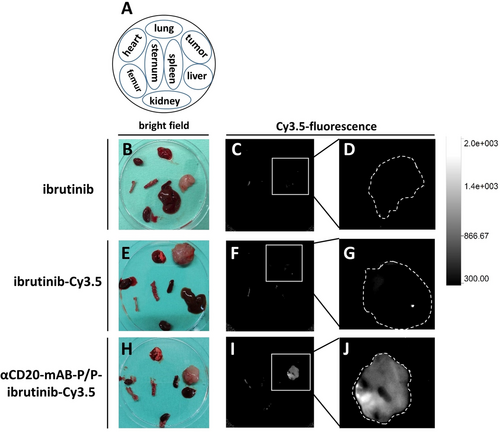
Biodistribution and tumor enrichment of αCD20-mAB-P/P-ibrutinib-Cy3.5 nanocarrier. A: Schematic overview of organs and tumors prepared from the treatment groups shown in B-J. Organs are always arranged in the same orientation (as depicted in the Scheme in A) in bright field (B, E, H) and red (Cy3.5) fluorescence (C–D, F–G, I–J). B–D: Ibrutinib-treated organs. E–G: ibrutinib-Cy3.5 treated organs. H–J: αCD20-mAB-P/P-ibrutinib-Cy3.5 treated organs. Significant Cy3.5-bound fluorescence signals were only detected in the tumor site, the fluorescence signal observed in the femur in all groups is caused by autofluorescence (I). D, G, J: Detailed analysis of tumors from C, F, I, respectively. The outer rim of the tumors were outlined (broken white line). Consistent fluorescence was only seen in tumors of the αCD20-mAB-P/P-ibrutinib-Cy3.5 treatment group. Scale in the upper panel represents arbitrary units of fluorescence. α, anti.
In patients, free ibrutinib (ImbruvicaR) is given orally at 560 mg per day, which would account for a dosage of 7.5 mg per kg for an average 75 kg adult. With due caution since parameters used in different experimental models cannot be directly compared, in previous preclinical mouse experiments, ibrutinib was applied orally in a dose from 6 mg kg−1,20 12 mg kg−1[21] or up to 50 mg kg−1[22] showing weak anti-lymphoma effects as a single drug in human xenograft and murine lymphoma models. Taking this dosage into account, ibrutinib was therefore applied in 410 to 3400 nanomol per single dose, respectively. In contrast, we applied only between 15 and 30 nanomol of ibrutinib-Cy3.5, targeted by 0.75–1.5 nanomol of nanocarrier as a systemic and parenteral single dose only twice a week, which is up to two orders of magnitude less than the untargeted ibrutinib applied orally. This results in much stronger anti-lymphoma effects as seen in the preclinical models cited before. After the treatments, clinical parameters for liver toxicity were recorded (Support. Figure 9), exhibiting significantly lower GOT and GPT values in αCD20-mAB-P/P-ibrutinib-Cy3.5 treated mice versus those treated with the untargeted ibrutinib, which points towards an effective concentration event of the drug in the tumor cells, but not other cells. Our new targeting and carrier system thereby confers transport of a novel anionic form of the BTK-inhibitor ibrutinib (ibrutinib-Cy3.5) into CD20-positive lymphoma cells in vitro and in vivo. The conjugate has the potential to spare the CD20-negative cells in vivo and therefore to concentrate the ibrutinib derivate specifically in CD20-expressing lymphoma cells and tumors. There it is internalized by CD20-receptor mediated endocytosis as known for type I αCD20-mABs23 and subsequently most likely undergoes endosomal maturation via protonation.24 Release first from the protamine and second from the endosome during this process by protamine-dependend endosomal destabilization events was also observed by others.25 However, this will be a subject of further investigations.
In vitro, this novel ibrutinib derivate thereby showed the same intracellular effects than unmodified ibrutinib, which means that the derivatization to a poly-anionic structure imposes no disadvantage in terms of reactivity towards the target cysteine in the BTK ATP binding pocket. Strikingly, in vivo, tumor growth was significantly inhibited when αCD20-mAB-P/P-ibrutinib-Cy3.5 was applied, while the same concentrations of unconjugated αCD20-mAB combined with free ibrutinib-Cy3.5 were not successful to slow tumor growth, which means that in vivo, it shows a major improvement of biotargeting of the ibrutinib compound towards tumor tissues. Of note, only tumors treated with the targeted conjugate αCD20-mAB-P/P-ibrutinib-Cy3.5 showed distinct detectable Cy3.5-fluorescence in ex vivo biodistribution studies, indicating that normal mouse organs were not affected and that the targeted nanocarrier system concentrated ibrutinib-Cy3.5 specifically within the tumor.
We found that the therapeutic effect of ibrutinib-Cy3.5 is imperatively dependent on its electrostatic assembly and delivery by assembly into the carrier antibody αCD20-mAB-P/P construct, which guides the kinase inhibitor to the intended target cells, concentrates it in the cells and amplifies its biological function at two orders of magnitude, according to the much lower dosages necessary. This observation strongly supports the new and unexpected macromolecular nanostructure as being necessary and sufficient for the in vitro and in vivo pharmacodynamic efficacy of our carrier system. Also, we found that the electrostatic complex between the ibrutinib-Cy3.5 polyanion and the antibody-protamine conjugate requires unconjugated protamine as an “electrostatic glue” between both components, leading to stable nanoparticles of a reproducible size distribution. For instance, when we purified the conjugate mixture from excess protamine (Figure 4 B and Support. Figure 7 B), the formation of the nanocarrier was not observed and the resulting component mixture was not effective in colony assays.
These findings are in contrast to those published for the unconjugated ibrutinib drug, which is given orally, which is known to affect and target cells independent of their origin, and which shows a 90 % irreversible absorption of given dosage by plasma proteins and more than 80 % rapid clearance and excretion mostly in faeces.26
Furthermore, it was observed that ibrutinib also binds to other kinases containing the similar reserved cysteine residue such as the epidermal growth factor receptor (EGFR), human EGFR 2 (HER2/neu), human EGFR 4 (HER4/ErbB4), interleukin-2-inducible T-cell kinase (ITK), and Janus kinase 3. As they also have a cysteine residue at analogous position27 inside the ATP-binding pocket, an ibrutinib binding to those will lead to considerable loss of available drug. As one example, ibrutinib displays significant BTK-independent effects on the T-cell lineage, which is in agreement with previous studies reporting that T-cell activation is blocked by irreversible binding of ibrutinib to ITK.13, 28 Our antibody-drug nanocarrier wraps anionic ibrutinib-Cy3.5 and transports it preferentially into CD20-positive cells. Since CD20-positive T-cells are extremely rare,29 one can assume that our transport system spares T-cells.
Conclusion
To our knowledge, this is the first time that an anionic drug-derivative assembles by electrostatic interactions with a cationic carrier system consisting of cationic protamine, which in turn was decorated with a cell target-specific antibody.
Our electrostatic assembly of drug-cargo to carrier-antibody imposes a number of major improvements: First, the multiplication of the cargo-to-carrier ratio, second the concentration of the drug at the desired place of action and last the selective process of molecular intervention. Moreover, our nanocarrier offers the possibility of being used as a platform technology with a broad target cell spectrum: a change of the targeting antibody from αCD20-mAB-protamine to αCD33-mAB-protamine would change the range of targeted cells from the B-cell lineage to myeloid cells in myeloid leukemia30, 31 and the change to αEGFR-mAB-protamine to solid tumor cells in lung cancer.32, 33 On the effector side, an exchange from ibrutinib-polyanion to a small molecular inhibitor with other pharmacodynamic properties could change the therapeutic warhead towards other pharmacological targets and modes of action.
Acknowledgements
We thank Mara Apel and Richard Holtmeier for excellent technical assistance. AF, RM, GL and WEB gratefully acknowledge the Deutsche Forschungsgemeinschaft DFG EXC 1003 Cluster of Excellence Cells in Motion for financial support, AF the Collaborative Research Centre (CRC) 1450–431460824 and CR the CRC 1009 (project B03) both funded by the DFG; SB and NB gratefully acknowledge the Interdisciplinary Centre for Clinical Research (IZKF Bäu2/009/19), CG the core unit PIX of IZKF for financial support, as well as PD (IZKF De2/006/20 and Collaborative Research Centre 1450C07A). The nanocarrier principle development was also supported by the Deutsche José Carreras Leukämie-Stiftung, DJCLS 04 R/2017 and DJCLS 04 R/2020 given to NB and SB, as well as DFG grant BA 6103/3-1 given to SB. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
AF, NB, LW, GL, and WEB have filed 2 patent applications on nanocarrier technology. The other authors declare no conflict of interest.