Formation of Short Zn−Zn Bonds Stabilized by Simple Cyanide and Isocyanide Ligands
Graphical Abstract
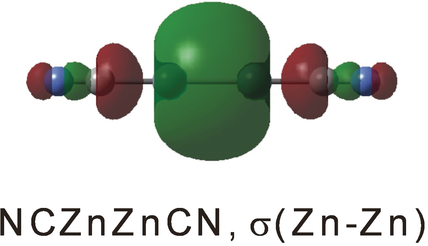
Cyanogen diluted in argon was reacted with laser-ablated Zn atoms to produce the NCZnCN and NCZnZnCN cyanides and their higher energy isocyanide counterparts, as well as ZnNC, which were isolated in excess argon at 4 K. CCSD(T) calculations find a short 2.367 Å Zn−Zn bond in the NCZnZnCN cyanide, and a short 2.347 Å Zn−Zn bond in the higher-energy isocyanide CNZnZnNC.
Abstract
Cyanogen diluted in argon was reacted with laser ablated Zn atoms to produce the NCZnCN and NCZnZnCN cyanides and higher energy isocyanides ZnNC, CNZnNC, and CNZnZnNC, which were isolated in excess argon at 4 K. These reaction products, identified from the matrix infrared spectra of their -CN and -NC chromophore ligand stretching modes, were confirmed by 13C and 15N isotopic substitution and comparison with frequencies calculated by the B3LYP and CCSD(T) methods using the all electron aug-cc-pVTZ basis sets. The cyanide and isocyanide products were increased markedly by mercury arc UV photolysis, which covers the zinc atomic absorption. The above electronic structure calculations that produce appropriate ligand frequencies for these dizinc products also provide their Zn−Zn bond lengths: CCSD(T) calculations find a short 2.367 Å Zn−Zn bond in the NCZnZnCN cyanide, a shorter 2.347 Å Zn−Zn bond in the 37.4 kJ mol−1 higher energy isocyanide CNZnZnNC, and a longer 4.024 Å bond in the dizinc van der Waals dimer. Thus, the diatomic cyanide (-CN) and isocyanide (-NC) ligands are as capable of stabilizing the Zn−Zn bond as many much larger ligands based on their measured and our calculated Zn−Zn bond lengths. This is the first example of dizinc complexes stabilized by different ligand isomers. Additional weaker bands in this region can be assigned to the analogous trizinc molecules NCZnZnZnCN and CNZnZnZnNC.
Conflict of interest
The authors declare no conflict of interest.