Acyclic Branched α-Fluoro Ketones for the Direct Asymmetric Mannich Reaction Leading to the Synthesis of β-Tetrasubstituted β-Fluoro Amines
Corresponding Author
Prof. Dr. Barry M. Trost
Department of Chemistry, Stanford University, 333 Campus Dr, Stanford, CA, 94305 USA
Search for more papers by this authorJacob S. Tracy
Department of Chemistry, Stanford University, 333 Campus Dr, Stanford, CA, 94305 USA
These authors contributed equally to this work.
Search for more papers by this authorTas Yusoontorn
Department of Chemistry, Stanford University, 333 Campus Dr, Stanford, CA, 94305 USA
These authors contributed equally to this work.
Search for more papers by this authorChao-I Joey Hung
Department of Chemistry, Stanford University, 333 Campus Dr, Stanford, CA, 94305 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Barry M. Trost
Department of Chemistry, Stanford University, 333 Campus Dr, Stanford, CA, 94305 USA
Search for more papers by this authorJacob S. Tracy
Department of Chemistry, Stanford University, 333 Campus Dr, Stanford, CA, 94305 USA
These authors contributed equally to this work.
Search for more papers by this authorTas Yusoontorn
Department of Chemistry, Stanford University, 333 Campus Dr, Stanford, CA, 94305 USA
These authors contributed equally to this work.
Search for more papers by this authorChao-I Joey Hung
Department of Chemistry, Stanford University, 333 Campus Dr, Stanford, CA, 94305 USA
Search for more papers by this authorGraphical Abstract
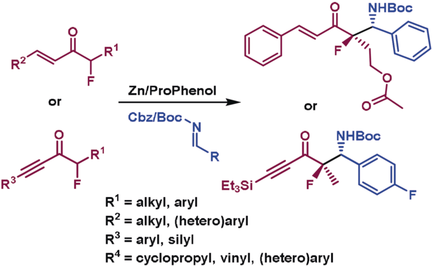
Break the cycle: Simple branched acyclic vinyl and alkynyl fluoro ketones undergo direct asymmetric Mannich reactions with aldimines to form β-fluoro amines bearing tetrasubstituted fluorine stereocenters. The zinc-ProPhenol-catalyzed transformation proceeds in excellent yield, and with high levels of diastereo- (up to <20:1) and enantioselectivity (up to 99 %).
Abstract
The preparation of acyclic β-fluoro amines bearing tetrasubstituted fluorine stereocenters is described via a direct Zn/ProPhenol-catalyzed Mannich reaction. The reaction utilizes branched vinyl or alkynyl α-fluoro ketones that can be coupled with a range of aryl, heteroaryl, vinyl, or cyclopropyl aldimines in high yield and with excellent diastereo- (up to >20:1) and enantioselectivity (up to 99 %). The use of readily cleaved tert-butoxycarbonyl (Boc) or carboxybenzyl (Cbz) imine protecting groups adds utility to the reaction by allowing for easy access to the free amine products under mild and chemoselective reaction conditions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201913927-sup-0001-misc_information.pdf6.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly, N. A. Meanwell, J. Med. Chem. 2015, 58, 8315.
- 2Y. Wang, R. Callejo, A. M. Z. Slawin, D. O'Hagan, Beilstein J. Org. Chem. 2014, 10, 18.
- 3M. Morgenthaler, E. Scweizer, A. Hoffman-Röder, F. Benini, R. E. Martin, G. Jaeschke, B. Wagner, H. Fischer, S. Bendels, D. Zummerli, J. Schneider, F. Diederich, M. Kansy, K. Müller, ChemMedChem 2007, 2, 1100.
- 4Q. A. Huchet, B. Kuhn, B. Wagner, H. Fischer, M. Kansy, D. Zimmerli, E. M. Carreira, K. Müller, J. Fluorine Chem. 2013, 152, 119.
- 5J. Y. Gauthier, N. Chauret, W. Cromlish, S. Desmarais, L. T. Duong, J. P. Falgueyret, D. B. Kimmel, S. Lamontagne, S. Léger, T. LeRiche, et al., Bioorg. Med. Chem. Lett. 2008, 18, 923.
- 6Y. Zhu, J. Han, J. Wang, N. Shibata, M. Sodeoka, V. A. Soloshonok, J. A. S. Coelho, F. D. Toste, Chem. Rev. 2018, 118, 3887.
- 7J. P. Maianti, H. Kanazawa, P. Dozzo, R. D. Matias, L. A. Feeney, E. S. Armstrong, D. J. Hildebrandt, T. R. Kane, M. J. Gliedt, A. A. Goldblum, M. S. Linsell, J. B. Aggen, J. Kondo, S. Hanessian, ACS Chem. Biol. 2014, 9, 2067.
- 8
- 8aC. D. Cox, P. J. Coleman, M. J. Breslin, D. B. Whitman, R. M. Garbaccio, M. E. Frley, C. A. Buser, E. S. Walsh, K. Hamilton, M. D. Schaber, et al., J. Med. Chem. 2008, 51, 4239;
- 8bC. D. Cox, M. J. Breslin, D. B. Whitman, P. J. Coleman, R. M. Gerbaccio, M. E. Fraley, M. M. Zrada, C. A. Buser, E. S. Walsh, K. Hamilton, et al., Bioorg. Med. Chem. Lett. 2007, 17, 2697;
- 8cE. J. Hicken, F. P. Marmsater, M. C. Munson, S. T. Schlachter, J. E. Robinson, S. Allen, L. E. Burgess, R. K. Delisle, J. P. Rizzi, G. T. Topalov, et al., ACS Med. Chem. Lett. 2014, 5, 78.
- 9S. P. Hameed, V. Patil, S. Solapure, U. Sharma, Madhavapeddia, A. Raichurkar, M. Chinnapattu, P. Manjrekar, G. Shanbhag, J. Puttur, et al., J. Med. Chem. 2014, 57, 4889.
- 10
- 10aDrugbank, structure search of approved, investigational, and experimental drugs containing a β-fluoro amine; https://www.drugbank.ca/ (accessed October 19, 2019). Polyfluorinated compounds at the beta-position are included in the results. D. S. Wishart et al., Nucleic Acids Res. 2018, 46, D 1074;
- 10bJ. L. Clark, L. Hollecker, J. C. Mason, L. J. Stuyver, P. M. Tharnish, S. Lostia, T. R. McBrayer, R. F. Schinazi, K. A. Watanabe, M. J. Otto, P. A. Furman, W. J. Stec, S. E. Patterson, K. W. Pankiewicz, J. Med. Chem. 2005, 48, 5504;
- 10cV. Peddie, M. Pietsch, K. M. Bromfield, R. N. Pike, P. J. Duggan, A. D. Abell, Synthesis 2010, 1845;
- 10dB. C. Barlaam, D. H. O'Donovan, S. J. Hughes, T. A. Moss, J. W. M. Nissink, J. S. Scott, B. Yang (AstraZeneca AB), US2018111931 A1, 2018.
- 11
- 11aZ. Jiang, Y. Pan, Y. Zhao, T. Ma, R. Lee, Y. Yang, K.-W. Huang, M. W. Wong, C.-H Tan, Angew. Chem. Int. Ed. 2009, 48, 3627; Angew. Chem. 2009, 121, 3681;
- 11bX. Han, J. Kwiatkowski, F. Xue, K.-W. Huang, Y. Lu, Angew. Chem. Int. Ed. 2009, 48, 7604; Angew. Chem. 2009, 121, 7740;
- 11cJ. H. Lee, D. Y. Kim, Synthesis 2010, 1860;
- 11dY. Pan, Y. Zhao, T. Ma, Y. Yang, H. Liu, Z. Jiang, C.-H. Tan, Chem. Eur. J. 2010, 16, 779;
- 11eS. J. Yoon, Y. K. Kang, D. Y. Kim, Synlett 2011, 420;
- 11fY. K. Kang, S. J. Yoon, D. Y. Kim, Bull. Korean Chem. Soc. 2011, 32, 1195;
- 11gY. K. Kang, D. Y. Kim, Tetrahedron Lett. 2011, 52, 2356;
- 11hM. Wasa, R. Y. Liu, S. P. Roche, E. N. Jacobsen, J. Am. Chem. Soc. 2014, 136, 12872;
- 11iH.-Y. Wang, K. Zhang, C.-W. Zheng, Z. Cui, D.-D. Cao, J.-X. Zhang, G. Zhao, Angew. Chem. Int. Ed. 2015, 54, 1775; Angew. Chem. 2015, 127, 1795;
- 11jE. Cosimi, O. D. Engl, J. Saadi, M.-O. Ebert, H. Wennemers, Angew. Chem. Int. Ed. 2016, 55, 13127; Angew. Chem. 2016, 128, 13321;
- 11kY. You, L. Zhang, L. Cui, X. Mi, S. Luo, Angew. Chem. Int. Ed. 2017, 56, 13814; Angew. Chem. 2017, 129, 14002.
- 12J. Kwiatkowski, Y. Lu, Org. Biomol. Chem. 2015, 13, 2350.
- 13
- 13aY. Pan, Y. Zhao, T. Ma, Y. Yang, H. Liu, Z. Jiang, C.-H. Tan, Chem. Eur. J. 2010, 16, 779;
- 13bM. Urban, M. Franc, M. Hofmanová, J. Vesely, Org. Biomol. Chem. 2017, 15, 9071.
- 14
- 14aL. Brewitz, F. Arteaga, A. Arteaga, L. Yin, K. Alagiri, N. Kumagai, M. Shibasaki, J. Am. Chem. Soc. 2015, 137, 15929;
- 14bL. Brewitz, N. Kumagai, M. Shibasaki, J. Fluorine Chem. 2017, 194, 1.
- 15
- 15aP. V. Balaji, L. Brewitz, N. Kumagai, M. Shibasaki, Angew. Chem. Int. Ed. 2019, 58, 2644; Angew. Chem. 2019, 131, 2670;
- 15bR. Ding, Z. A. De los Santos, C. Wolf, ACS Catal. 2019, 9, 2169.
- 16For related work on the Henry reaction with branched α-fluoro nitro compounds see: B. A. Vara, J. N. Johnston, J. Am. Chem. Soc. 2016, 138, 13794.
- 17
- 17aY. Zhao, Y. Pan, H. Liu, Y. Yang, Z. Jiang, C.-H. Tan, Chem. Eur. J. 2011, 17, 3571;
- 17bB. M. Trost, T. Saget, A. Lerchen, C.-I. Hung, Angew. Chem. Int. Ed. 2016, 55, 781; Angew. Chem. 2016, 128, 791;
- 17cV. Vaithiyanathan, M. J. Kim, Y. Liu, H. Yan, C. E. Song, Chem. Eur. J. 2017, 23, 1268.
- 18B. M. Trost, C.-I. J. Hung, G. Mata, Angew. Chem. Int. Ed. 2019, https://doi.org/10.1002/anie.201909692; Angew. Chem. 2019, https://doi.org/10.1002/ange.201909692.
- 19B. M. Trost, J. S. Tracy, T. Yusoontorn, Org. Lett. 2019, 21, 1207.
- 20B. M. Trost, C.-I. J. Hung, D. C. Koester, Y. Miller, Org. Lett. 2015, 17, 3778.
- 21For examples of ProPhenol-catalyzed alkyne addition reactions see:
- 21aB. M. Trost, A. H. Weiss, A. J. von Wangelin, J. Am. Chem. Soc. 2006, 128, 8;
- 21bB. M. Trost, M. J. Bartlett, A. H. Weiss, A. J. von Wangelin, V. S. Chan, Chem. Eur. J. 2012, 18, 16498;
- 21cB. M. Trost, A. Quintard, Angew. Chem. Int. Ed. 2012, 51, 6704; Angew. Chem. 2012, 124, 6808;
- 21dB. M. Trost, H. Gholami, J. Am. Chem. Soc. 2018, 140, 11623.
- 22CCDC 1962584 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 23
- 23aG.-P. Lu, K. R. Voigtritter, C. Cai, B. H. Lipshutz, J. Org. Chem. 2012, 77, 3700.
- 23bFor an example of these conditions used for retention of olefin geometry in Suzuki cross-coupling of alpha-halo enones see: B. M. Trost, J. S. Tracy, ACS Catal. 2019, 9, 1584.
- 24B. M. Trost, C.-I. J. Hung, E. Gnanamani, ACS Catal. 2019, 9, 1549.