Catalytic Selective Dihydrosilylation of Internal Alkynes Enabled by Rare-Earth Ate Complex
Wufeng Chen
State Key Laboratory of Elemento-Organic Chemistry and College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorDr. Haibin Song
State Key Laboratory of Elemento-Organic Chemistry and College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Dr. Jianfeng Li
State Key Laboratory of Elemento-Organic Chemistry and College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Chunming Cui
State Key Laboratory of Elemento-Organic Chemistry and College of Chemistry, Nankai University, Tianjin, 300071 China
Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin, 300072 China
Search for more papers by this authorWufeng Chen
State Key Laboratory of Elemento-Organic Chemistry and College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorDr. Haibin Song
State Key Laboratory of Elemento-Organic Chemistry and College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Dr. Jianfeng Li
State Key Laboratory of Elemento-Organic Chemistry and College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Chunming Cui
State Key Laboratory of Elemento-Organic Chemistry and College of Chemistry, Nankai University, Tianjin, 300071 China
Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin, 300072 China
Search for more papers by this authorGraphical Abstract
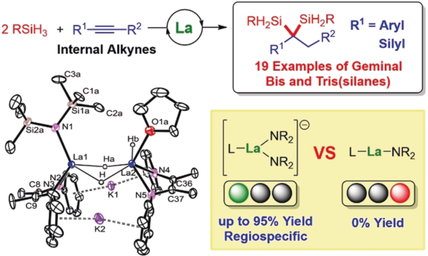
Big metal magic: Dihydrosilylation of aryl- and silyl-substituted internal alkynes is successfully achieved by a lanthanum bis(amido) ate complex to yield geminal bis- and tris(silanes), respectively. The high activity could be attributed to the large lanthanum ion and anionic nature of the catalyst.
Abstract
Hydrosilylation of alkynes generally yield vinylsilanes, which are inert to the further hydrosilylation because of the steric effects. Reported here is the first successful dihydrosilylation of aryl- and silyl-substituted internal alkynes enabled by a rare-earth ate complex to yield geminal bis- and tris(silanes), respectively. The lanthanum bis(amido) ate complex supported by an ene-diamido ligand proved to be the ideal catalyst for this unprecedented transformation, while the same series of yttrium and samarium alkyl and samarium bis(amido) ate complexes exhibited poor activity and selectivity, indicating significant effects of the ionic size and ate structure of the rare-earth catalysts.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201913773-sup-0001-misc_information.pdf3.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 The Chemistry of Organic Silicon Compounds, Vol. 2 (Eds.: ), Wiley, New York, 1998, p. 1687.
10.1002/0470857250.ch29 Google Scholar
- 2
- 2aL. Gao, Y. Zhang, Z. Song, Synlett 2013, 24, 139–144;
- 2bD. R. Williams, A. I. Morales-Ramos, C. M. Williams, Org. Lett. 2006, 8, 4393–4396;
- 2cZ. Yin, Z. Liu, Z. Huang, Y. Chu, Z. Chu, J. Hu, L. Gao, Z. Song, Org. Lett. 2015, 17, 1553–1556;
- 2dL. Li, Y. Chu, L. Gao, Z. Song, Chem. Commun. 2015, 51, 15546–15549;
- 2eZ. Liu, X. Lin, N. Yang, Z. Su, C. Hu, P. Xiao, Y. He, Z. Song, J. Am. Chem. Soc. 2016, 138, 1877–1883;
- 2fZ. Chu, K. Wang, L. Gao, Z. Song, Chem. Commun. 2017, 53, 3078–3081;
- 2gY. Zhang, Q. Guo, X. Sun, J. Lu, Y. Cao, Q. Pu, Z. Chu, L. Gao, Z. Song, Angew. Chem. Int. Ed. 2018, 57, 942–946; Angew. Chem. 2018, 130, 954–958.
- 3
- 3aC. Eaborn, J. D. Smith, J. Chem. Soc. Dalton Trans. 2001, 1541–1552;
- 3bC. Eaborn, J. Chem. Soc. Dalton Trans. 2001, 3397–3406;
- 3cA. Schnepf, R. Köppe, H. Schnöckel, Angew. Chem. Int. Ed. 2001, 40, 1241–1243;
10.1002/1521-3773(20010401)40:7<1241::AID-ANIE1241>3.0.CO;2-J CAS PubMed Web of Science® Google ScholarAngew. Chem. 2001, 113, 1287–1290;
- 3dA. C. Jones, A. W. Sanders, W. H. Sikorski, K. L. Jansen, H. J. Reich, J. Am. Chem. Soc. 2008, 130, 6060–6061.
- 4R. J. P. Corriu, M. Granier, G. Lanneau, J. Organomet. Chem. 1998, 562, 79–88.
- 5Z. Liu, H. Tan, T. Fu, Y. Xia, D. Qiu, Y. Zhang, J. Wang, J. Am. Chem. Soc. 2015, 137, 12800–12803.
- 6H. Hazrati, M. Oestreich, Org. Lett. 2018, 20, 5367–5369.
- 7
- 7aJ. Liedtke, S. Loss, C. Widauer, H. Grützmacher, Tetrahedron 2000, 56, 143–156;
- 7bP.-F. Fu, J. Mol. Catal. A 2006, 243, 253–257;
- 7cA. Gorczyński, M. Zaranek, S. Witomska, A. Bocian, A. R. Stefankiewicz, M. Kubicki, V. Patroniak, P. Pawluć, Catal. Commun. 2016, 78, 71–74.
- 8
- 8aB. M. Trost, Z. T. Ball, Synthesis 2005, 853–887;
- 8bB. Marciniec, H. Maciejewski, C. Pietraszuk, P. Pawluć in Hydrosilylation: A Comprehensive Review on Recent Advances, Vol. 1 (Ed.: ), Springer, Berlin, 2009, chap. 2 and 3;
10.1007/978-1-4020-8172-9 Google Scholar
- 8cD. S. W. Lim, E. A. Anderson, Synthesis 2012, 44, 983–1010;
- 8dY. Nakajima, S. Shimada, RSC Adv. 2015, 5, 20603–20616;
- 8eJ. Sun, L. Deng, ACS Catal. 2016, 6, 290–300.
- 9M.-Y. Hu, J. Lian, W. Sun, T.-Z. Qiao, S.-F. Zhu, J. Am. Chem. Soc. 2019, 141, 4579–4583.
- 10
- 10aJ. Li, C. Zhao, J. Liu, H. Huang, F. Wang, X. Xu, C. Cui, Inorg. Chem. 2016, 55, 9105–9111;
- 10bJ. Liu, W. Chen, J. Li, C. Cui, ACS Catal. 2018, 8, 2230–2235.
- 11
- 11aT. V. Mahrova, G. K. Fukin, A. V. Cherkasov, A. A. Trifonov, N. Ajellal, J.-F. Carpentier, Inorg. Chem. 2009, 48, 4258–4266;
- 11bT. K. Panda, H. Kaneko, K. Pal, H. Tsurugi, K. Mashima, Organometallics 2010, 29, 2610–2615.
- 12Y. Bai, W. Chen, J. Li, C. Cui, Coord. Chem. Rev. 2019, 383, 132–154.
- 13
- 13aG. R. Giesbrecht, G. D. Whitener, J. Arnold, J. Chem. Soc. Dalton Trans. 2001, 923–927;
- 13bD. V. Vitanova, F. Hampel, K. C. Hultzsch, J. Organomet. Chem. 2005, 690, 5182–5197;
- 13cA. N. Kornev, V. V. Sushev, Y. S. Panova, N. V. Belina, O. V. Lukoyanova, G. K. Fukin, S. Y. Ketkov, G. A. Abakumov, P. Lönnecke, E. Hey-Hawkins, Inorg. Chem. 2012, 51, 874–881;
- 13dF. Wang, Y. Wei, S. Wang, X. Zhu, S. Zhou, G. Yang, X. Gu, G. Zhang, X. Mu, Organometallics 2015, 34, 86–93.
- 14T. Gans-Eichler, D. Gudat, K. Nättinen, M. Nieger, Chem. Eur. J. 2006, 12, 1162–1173.
- 15A. A. Kissel, T. V. Mahrova, D. M. Lyubov, A. V. Cherkasov, G. K. Fukin, A. A. Trifonov, I. Del Rosal, L. Maron, Dalton Trans. 2015, 44, 12137–12148.
- 16
- 16aG. A. Molander, J. A. C. Romero, Chem. Rev. 2002, 102, 2161–2186;
- 16bD. Liu, B. Liu, Z. Pan, J. Li, C. Cui, Sci. China Chem. 2019, 62, 571–682.
- 17
- 17aB. B. Snider, M. Karras, R. S. E. Conn, J. Am. Chem. Soc. 1978, 100, 4624–4626;
- 17bY. Gao, F. Sato, J. Chem. Soc. Chem. Commun. 1995, 659–660;
- 17cE. Shirakawa, T. Yamagami, T. Kimura, S. Yamaguchi, T. Hayashi, J. Am. Chem. Soc. 2005, 127, 17164–17165;
- 17dB. H. Tan, J. Dong, N. Yoshikai, Angew. Chem. Int. Ed. 2012, 51, 9610–9614; Angew. Chem. 2012, 124, 9748–9752;
- 17eY. H. Lee, B. Morandi, Angew. Chem. Int. Ed. 2019, 58, 6444–6448; Angew. Chem. 2019, 131, 6510–6515.
- 18
- 18aJ. Oyamada, M. Nishiura, Z. Hou, Angew. Chem. Int. Ed. 2011, 50, 10720–10723; Angew. Chem. 2011, 123, 10908–10911;
- 18bD. Liu, C. Yao, R. Wang, M. Wang, Z. Wang, C. Wu, F. Lin, S. Li, X. Wan, D. Cui, Angew. Chem. Int. Ed. 2015, 54, 5205–5209; Angew. Chem. 2015, 127, 5294–5298.
- 19
- 19aG. Jeske, H. Lauke, H. Mauermann, P. N. Swepston, H. Schumann, T. J. Marks, J. Am. Chem. Soc. 1985, 107, 8091–8103;
- 19bC. Wang, L. Xiang, X. Leng, Y. Chen, Dalton Trans. 2017, 46, 1218–1227.
- 20
- 20aS. Bambirra, M. W. Bouwkamp, A. Meetsma, B. Hessen, J. Am. Chem. Soc. 2004, 126, 9182–9183;
- 20bC. G. J. Tazelaar, S. Bambirra, D. van Leusen, A. Meetsma, B. Hessen, J. H. Teuben, Organometallics 2004, 23, 936–939;
- 20cS. Bambirra, A. Meetsma, B. Hessen, A. P. Bruins, Organometallics 2006, 25, 3486–3495.
- 21A standing mixture of 3 and 1.5 equivalents of nC6H13SiH3 in n-hexane at room temperature overnight could result in the formation of single crystals of 10 and 9, but no suitable single crystals for X-ray diffraction can been obtained in toluene.
- 22CCDC 1951111 (2), 1921811 (3), and 1946993 (10) contain the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre