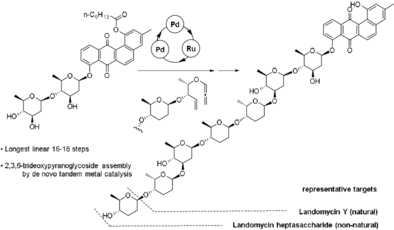
Flexible Total Synthesis of 11-Deoxylandomycins and Their Non-Natural Analogues by Way of Asymmetric Metal Catalysis
Juyeol Lee
Department of Chemistry, Pohang University of Science and Technology (POSTECH), Pohang, 37673 Republic of Korea
Search for more papers by this authorJihun Kang
Department of Chemistry, Pohang University of Science and Technology (POSTECH), Pohang, 37673 Republic of Korea
Search for more papers by this authorSukhyun Lee
Department of Chemistry, Pohang University of Science and Technology (POSTECH), Pohang, 37673 Republic of Korea
Search for more papers by this authorCorresponding Author
Prof. Young Ho Rhee
Department of Chemistry, Pohang University of Science and Technology (POSTECH), Pohang, 37673 Republic of Korea
Search for more papers by this authorJuyeol Lee
Department of Chemistry, Pohang University of Science and Technology (POSTECH), Pohang, 37673 Republic of Korea
Search for more papers by this authorJihun Kang
Department of Chemistry, Pohang University of Science and Technology (POSTECH), Pohang, 37673 Republic of Korea
Search for more papers by this authorSukhyun Lee
Department of Chemistry, Pohang University of Science and Technology (POSTECH), Pohang, 37673 Republic of Korea
Search for more papers by this authorCorresponding Author
Prof. Young Ho Rhee
Department of Chemistry, Pohang University of Science and Technology (POSTECH), Pohang, 37673 Republic of Korea
Search for more papers by this authorGraphical Abstract
Abstract
A de novo first collective total synthesis of 11-deoxylandomycins is reported. A signature step is featured by the Pd-catalyzed asymmetric addition of alcohol to ene-alkoxyallenes that assembles oligomeric 2,3,6-trideoxyoligosaccharides. The unique feature of the protocol is illustrated by a flexible access to various natural 11-deoxylandomycins as well as non-natural analogues.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201913706-sup-0001-misc_information.pdf5.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. Rohr, R. Thiericke, Nat. Prod. Rep. 1992, 9, 103–137.
- 2R. T. Crow, B. Rosenbaum, R. Smith, Y. Guo, K. S. Ramos, G. A. Sulikowski, Bioorg. Med. Chem. Lett. 1999, 9, 1663–1666.
- 3K. A. Shaaban, S. Srinivasan, R. Kumar, C. Damodaran, J. Rohr, J. Nat. Prod. 2011, 74, 2–11.
- 4K. A. Shaaban, C. Stamatkin, C. Damodaran, J. Rohr, J. Antibiot. 2011, 64, 141–150.
- 5
- 5aA. Luzhetskyy, T. Liu, M. Fedoryshyn, B. Ostash, V. Fedorenko, J. Rohr, A. Bechthold, ChemBioChem 2004, 5, 1567–1570;
- 5bL. Zhu, A. Luzhetskyy, M. Luzhetska, C. Mattingly, V. Adams, A. Bechthold, J. Rohr, ChemBioChem 2007, 8, 83–88.
- 6For related studies on the synthesis of Landomycin A in which d-amicetose (D-ring) is replaced by d-olivose, see:
- 6aX. Yang, B. Fu, B. Yu, J. Am. Chem. Soc. 2011, 133, 12433–12435;
- 6bW. R. Roush, C. E. Bennett, J. Am. Chem. Soc. 2000, 122, 6124–6125;
- 6cH. Tanaka, S. Yamaguchi, A. Yoshizawa, M. Takagi, K. Shin-ya, T. Takahashi, Chem. Asian J. 2010, 5, 1407–1424;
- 6dY. Guo, G. A. Sulikowski, J. Am. Chem. Soc. 1998, 120, 1392–1397;
- 6eS. Yalamanchili, D. Lloyd, C. S. Bennett, Org. Lett. 2019, 21, 3674–3677.
- 7For selected review on the synthesis of 2-deoxyglycosides, see:
- 7aD. Hou, T. L. Lowary, Carbohydr. Res. 2009, 344, 1911–1940;
- 7bA. Borovika, P. Nagorny, J. Carbohydr. Chem. 2012, 31, 255–283;
- 7cC. S. Bennett, M. C. Galan, Chem. Rev. 2018, 118, 7931–7985;
- 7dA. Z. Aljahdali, P. Shi, Y. Zhong, G. A. O'Doherty, Adv. Carbohydr. Chem. Biochem., Vol. 69 (Ed.: ), Academic Press, San Diego, 2013, pp. 55–123.
- 8For selected review on the synthesis of natural product containing deoxyglycoside, see:
- 8aB. Yu, J. Sun, X. Yang, Acc. Chem. Res. 2012, 45, 1227–1236;
- 8bY. Yang, X. Zhang, B. Yu, Nat. Prod. Rep. 2015, 32, 1331–1355;
- 8cK. Kitamura, Y. Ando, T. Matsumoto, K. Suzuki, Chem. Rev. 2018, 118, 1495–1598.
- 9For synthetic methods allowing the assembly of 2,3,6-trideoxyoligosaccharides in a sequential manner, see:
- 9aW. Song, Y. Zhao, J. C. Lynch, H. Kim, W. Tang, Chem. Commun. 2015, 51, 17475–17478;
- 9bR. S. Babu, M. Zhou, G. A. O'Doherty, J. Am. Chem. Soc. 2004, 126, 3428–3429.
- 10
- 10aW. Lim, J. Kim, Y. H. Rhee, J. Am. Chem. Soc. 2014, 136, 13618–13621;
- 10bM. Kim, S. Kang, Y. H. Rhee, Angew. Chem. Int. Ed. 2016, 55, 9733–9737; Angew. Chem. 2016, 128, 9885–9889;
- 10cJ. Lee, S. Kang, J. Kim, D. Moon, Y. H. Rhee, Angew. Chem. Int. Ed. 2019, 58, 628–631; Angew. Chem. 2019, 131, 638–641.
- 11K. A. Parker, Q.-j. Ding, Tetrahedron 2000, 56, 10249–10254.
- 12
- 12aJ. P. Issa, C. S. Bennett, J. Am. Chem. Soc. 2014, 136, 5740–5744;
- 12bD. Lloyd, M. Bylsma, D. K. Bright, X. Chen, C. S. Bennett, J. Org. Chem. 2017, 82, 3926–3934;
- 12cD. Lloyd, C. S. Bennett, Chem. Eur. J. 2018, 24, 7610–7614.
- 13For details on the analysis of spectral data that confirms the regio- and stereoselectivity, see the Supporting Information.
- 14Unlike the glycosyl halides that are used in Yu's total synthesis of Landomycin A, only trace amount of glycal was observed in this reaction. For this issue, see ref. [6a]. Also see: X. Yang, B. Yu, Synthesis 2016, 48, 1693–1699.
- 15H. Sajiki, K. Hirota, Tetrahedron 1998, 54, 13981–13996.
- 16The 13C spectra revealed discrepancy in the chemical shift of C7a from the literature value reported in ref. [3]. The observed chemical shift is consistent with all the synthetic derivatives reported in this work as well as with the rest of 11-deoxylandomycin members isolated from natural substances.
- 17H. Kim, H. Men, C. Lee, J. Am. Chem. Soc. 2004, 126, 1336–1337.