Synthesis and Target Identification of Benzoxepane Derivatives as Potential Anti-Neuroinflammatory Agents for Ischemic Stroke
Cheng-Long Gao
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Zhangjiang Hi-Tech Park, Shanghai, 201203 China
State Key Laboratory of Natural Medicines, Jiangsu Key Laboratory of Drug Screening, China Pharmaceutical University, #24 Tong Jia Xiang Street, Nanjing, 210009 China
These authors contributed equally to this work.
Search for more papers by this authorProf. Gui-Ge Hou
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Zhangjiang Hi-Tech Park, Shanghai, 201203 China
School of Pharmacy, The Key Laboratory of Prescription Effect and Clinical Evaluation of State Administration of Traditional Chinese Medicine of China, Binzhou Medical University, Yantai, 264003 China
These authors contributed equally to this work.
Search for more papers by this authorJin Liu
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Zhangjiang Hi-Tech Park, Shanghai, 201203 China
These authors contributed equally to this work.
Search for more papers by this authorTong Ru
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Zhangjiang Hi-Tech Park, Shanghai, 201203 China
Search for more papers by this authorDr. Ya-Zhou Xu
State Key Laboratory of Natural Medicines, Jiangsu Key Laboratory of Drug Screening, China Pharmaceutical University, #24 Tong Jia Xiang Street, Nanjing, 210009 China
Search for more papers by this authorShun-Yi Zhao
State Key Laboratory of Natural Medicines, Jiangsu Key Laboratory of Drug Screening, China Pharmaceutical University, #24 Tong Jia Xiang Street, Nanjing, 210009 China
Search for more papers by this authorProf. Hui Ye
State Key Laboratory of Natural Medicines, Jiangsu Key Laboratory of Drug Screening, China Pharmaceutical University, #24 Tong Jia Xiang Street, Nanjing, 210009 China
Search for more papers by this authorProf. Lu-Yong Zhang
State Key Laboratory of Natural Medicines, Jiangsu Key Laboratory of Drug Screening, China Pharmaceutical University, #24 Tong Jia Xiang Street, Nanjing, 210009 China
Search for more papers by this authorProf. Kai-Xian Chen
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Zhangjiang Hi-Tech Park, Shanghai, 201203 China
Open Studio for Druggability Research of Marine Natural Products, Pilot National Laboratory for Marine Science and Technology (Qingdao), 1 Wenhai Road, Aoshanwei, Jimo, Qingdao, 266237 China
Search for more papers by this authorCorresponding Author
Prof. Yue-Wei Guo
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Zhangjiang Hi-Tech Park, Shanghai, 201203 China
Open Studio for Druggability Research of Marine Natural Products, Pilot National Laboratory for Marine Science and Technology (Qingdao), 1 Wenhai Road, Aoshanwei, Jimo, Qingdao, 266237 China
Search for more papers by this authorCorresponding Author
Prof. Tao Pang
State Key Laboratory of Natural Medicines, Jiangsu Key Laboratory of Drug Screening, China Pharmaceutical University, #24 Tong Jia Xiang Street, Nanjing, 210009 China
Search for more papers by this authorCorresponding Author
Prof. Xu-Wen Li
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Zhangjiang Hi-Tech Park, Shanghai, 201203 China
Open Studio for Druggability Research of Marine Natural Products, Pilot National Laboratory for Marine Science and Technology (Qingdao), 1 Wenhai Road, Aoshanwei, Jimo, Qingdao, 266237 China
Search for more papers by this authorCheng-Long Gao
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Zhangjiang Hi-Tech Park, Shanghai, 201203 China
State Key Laboratory of Natural Medicines, Jiangsu Key Laboratory of Drug Screening, China Pharmaceutical University, #24 Tong Jia Xiang Street, Nanjing, 210009 China
These authors contributed equally to this work.
Search for more papers by this authorProf. Gui-Ge Hou
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Zhangjiang Hi-Tech Park, Shanghai, 201203 China
School of Pharmacy, The Key Laboratory of Prescription Effect and Clinical Evaluation of State Administration of Traditional Chinese Medicine of China, Binzhou Medical University, Yantai, 264003 China
These authors contributed equally to this work.
Search for more papers by this authorJin Liu
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Zhangjiang Hi-Tech Park, Shanghai, 201203 China
These authors contributed equally to this work.
Search for more papers by this authorTong Ru
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Zhangjiang Hi-Tech Park, Shanghai, 201203 China
Search for more papers by this authorDr. Ya-Zhou Xu
State Key Laboratory of Natural Medicines, Jiangsu Key Laboratory of Drug Screening, China Pharmaceutical University, #24 Tong Jia Xiang Street, Nanjing, 210009 China
Search for more papers by this authorShun-Yi Zhao
State Key Laboratory of Natural Medicines, Jiangsu Key Laboratory of Drug Screening, China Pharmaceutical University, #24 Tong Jia Xiang Street, Nanjing, 210009 China
Search for more papers by this authorProf. Hui Ye
State Key Laboratory of Natural Medicines, Jiangsu Key Laboratory of Drug Screening, China Pharmaceutical University, #24 Tong Jia Xiang Street, Nanjing, 210009 China
Search for more papers by this authorProf. Lu-Yong Zhang
State Key Laboratory of Natural Medicines, Jiangsu Key Laboratory of Drug Screening, China Pharmaceutical University, #24 Tong Jia Xiang Street, Nanjing, 210009 China
Search for more papers by this authorProf. Kai-Xian Chen
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Zhangjiang Hi-Tech Park, Shanghai, 201203 China
Open Studio for Druggability Research of Marine Natural Products, Pilot National Laboratory for Marine Science and Technology (Qingdao), 1 Wenhai Road, Aoshanwei, Jimo, Qingdao, 266237 China
Search for more papers by this authorCorresponding Author
Prof. Yue-Wei Guo
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Zhangjiang Hi-Tech Park, Shanghai, 201203 China
Open Studio for Druggability Research of Marine Natural Products, Pilot National Laboratory for Marine Science and Technology (Qingdao), 1 Wenhai Road, Aoshanwei, Jimo, Qingdao, 266237 China
Search for more papers by this authorCorresponding Author
Prof. Tao Pang
State Key Laboratory of Natural Medicines, Jiangsu Key Laboratory of Drug Screening, China Pharmaceutical University, #24 Tong Jia Xiang Street, Nanjing, 210009 China
Search for more papers by this authorCorresponding Author
Prof. Xu-Wen Li
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Zhangjiang Hi-Tech Park, Shanghai, 201203 China
Open Studio for Druggability Research of Marine Natural Products, Pilot National Laboratory for Marine Science and Technology (Qingdao), 1 Wenhai Road, Aoshanwei, Jimo, Qingdao, 266237 China
Search for more papers by this authorGraphical Abstract
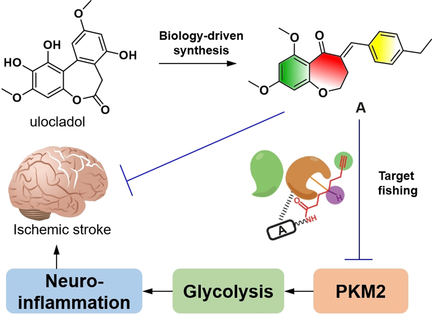
Fishing around: The benzoxepane derivative A was effective in vivo, ameliorating both sickness behavior through anti-inflammation in LPS-induced neuroinflammatory mice model and cerebral ischemic injury through anti-neuroinflammation in rats subjected to transient middle cerebral artery occlusion. Target fishing identified PKM2 as the target protein for A. Furthermore, A exhibited an anti-neuroinflammatory effect in vitro and in vivo by inhibiting PKM2-mediated glycolysis and NLRP3 activation.
Abstract
Benzoxepane derivatives were designed and synthesized, and one hit compound emerged as being effective in vitro with low toxicity. In vivo, this hit compound ameliorated both sickness behavior through anti-inflammation in LPS-induced neuroinflammatory mice model and cerebral ischemic injury through anti-neuroinflammation in rats subjected to transient middle cerebral artery occlusion. Target fishing for the hit compound using photoaffinity probes led to identification of PKM2 as the target protein responsible for anti-inflammatory effect of the hit compound. Furthermore, the hit exhibited an anti-neuroinflammatory effect in vitro and in vivo by inhibiting PKM2-mediated glycolysis and NLRP3 activation, indicating PKM2 as a novel target for neuroinflammation and its related brain disorders. This hit compound has a better safety profile compared to shikonin, a reported PKM2 inhibitor, identifying it as a lead compound in targeting PKM2 for the treatment of inflammation-related diseases.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201912489-sup-0001-misc_information.pdf6.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1G.-J. Hankey, Lancet 2017, 389, 641–654.
- 2Z.-H. Zhou, J.-F. Lu, W.-W. Li, A. Manaenko, X.-H. Hou, Q.-Y. Mei, J.-L. Huang, J.-P. Tang, J.-H. Zhang, H.-H. Yao, Q. Hu, Pharmacol. Ther. 2018, 191, 23–42.
- 3Y. Fu, Q. Liu, J. Anrather, F.-D. Shi, Nat. Rev. Neurol. 2015, 11, 524–535.
- 4X. Hu, P. Li, Y. Guo, H. Wang, R.-K. Leak, S. Chen, Y. Gao, J. Chen, Stroke 2012, 43, 3063–3070.
- 5X.-M. Hu, R.-K. Leak, Y.-J. Shi, J. Suenaga, Y.-Q. Gao, P. Zheng, J. Chen, Nat. Rev. Neurol. 2015, 11, 56–64.
- 6L.-C. Yang, M. Xie, M.-H. Yang, Y. Yu, S. Zhu, W. Hou, R. Kang, M.-T. Lotze, T.-R. Billiar, H.-C. Wang, L.-Z. Cao, D.-L. Tang, Nat. Commun. 2014, 5, 4436.
- 7B. Zhang, H. Chen, J. Ouyang, Y. Xie, L. Chen, Q. Tan, X. Du, N. Su, Z. Ni, L. Chen, Autophagy 2019, DOI: 10.1080/15548627.2019.1664705.
- 8A. Christoforow, J. Wilke, A. Binici, A. Pahl, C. Ostermann, S. Sievers, H. Waldmann, Angew. Chem. Int. Ed. 2019, 58, 14715–14723; Angew. Chem. 2019, 131, 14857–14865.
- 9M. Engler, T. Anke, O. Sterner, U. Brandt, J. Antibiot. 1997, 50, 325–329.
- 10G.-R. Pettit, A. Numata, C. Iwamoto, Y. Usami, T. Yamada, H. Ohishi, G.-M. Cragg, J. Nat. Prod. 2006, 69, 323–327.
- 11U. Höller, G.-M. König, A.-D. Wright, Eur. J. Org. Chem. 1999, 2949–2955.
10.1002/(SICI)1099-0690(199911)1999:11<2949::AID-EJOC2949>3.0.CO;2-Y CAS Web of Science® Google Scholar
- 12K. Chakraborty, V.-K. Raola, Nat. Prod. Res. 2019, 33, 2329–2337.
- 13Y. Nagai, A. Irie, H. Nakamura, K. Hino, H. Uno, H. Nishimura, J. Med. Chem. 1982, 25, 1065–1070.
- 14M. Kurokawa, H. Uno, H. Nakamura, F. Sato, S. Naruto, J. Med. Chem. 1990, 33, 504–509.
- 15
- 15aG. Li, H. Li, W. Tang, Y.-W. Guo, X.-W. Li, Org. Lett. 2019, 21, 5660–5664;
- 15bM. Yang, H. Li, Q. Zhang, Q.-H. Wu, G. Li, K.-X. Chen, Y.-W. Guo, W. Tang, X.-W. Li, Bioorg. Med. Chem. 2019, 27, 3469–3476;
- 15cJ. Liu, H. Li, K.-X. Chen, J.-P. Zuo, Y.-W. Guo, W. Tang, X.-W. Li, J. Med. Chem. 2018, 61, 11298–11308;
- 15dF. Ye, J. Li, Y. Wu, Z.-D. Zhu, E. Mollo, M. Gavagnin, Y.-C. Gu, W.-L. Zhu, X.-W. Li, Y.-W. Guo, Org. Lett. 2018, 20, 2637–2640;
- 15eF. Ye, Z.-D. Zhu, J.-S. Chen, J. Li, Y.-C. Gu, W.-L. Zhu, X.-W. Li, Y.-W. Guo, Org. Lett. 2017, 19, 4183–4186.
- 16
- 16aD.-S. Zhu, H.-J. Guo, Y. Chang, Y. Ni, L. Li, Z.-M. Zhang, P.-L. Hao, Y. Xu, K. Ding, Z.-Q. Li, Angew. Chem. Int. Ed. 2018, 57, 9284–9289; Angew. Chem. 2018, 130, 9428–9433;
- 16bZ.-Q. Li, D.-Y. Wang, L. Li, S.-J. Pan, Z.-K. Na, C.-Y.-J. Tan, S.-Q. Yao, J. Am. Chem. Soc. 2014, 136, 9990–9998;
- 16cZ.-Q. Li, P.-L. Hao, L. Li, C.-Y.-J. Tan, X.-M. Cheng, G.-Y.-J. Chen, S.-K. Sze, H.-M. Shen, S.-Q. Yao, Angew. Chem. Int. Ed. 2013, 52, 8551–8556; Angew. Chem. 2013, 125, 8713–8718.
- 17M. Xie, Y. Yu, R. Kang, S. Zhu, L.-C. Yang, L. Zeng, X.-F. Sun, M.-H. Yang, Nat. Commun. 2016, 7, 13280.
- 18N. Arichi, K.-I. Yamada, Y. Yamaoka, K. Takasu, J. Am. Chem. Soc. 2015, 137, 9579–9582.
- 19W.-W. Chen, X. Zhang, W.-J. Huang, Mol. Med. Rep. 2016, 13, 3391–3396.
- 20M. Pennisi, R. Crupi, D. Paola, R. M.-L. Ontario, R. Bella, E.-J. Calabrese, R. Crea, S. Cuzzocrea, V. Calabrese, J. Neurosci. Res. 2017, 95, 1360–1372.
- 21A. Alawieh, E.-F. Langley, S. Weber, D.-A. Adkins, S. Tomlinson, J. Neurosci. 2018, 38, 2519–2532.
- 22R. Guruswamy, A. Elali, Int. J. Mol. Sci. 2017, 18, 496.
- 23Y. Lee, S.-R. Lee, S.-S. Choi, H.-G. Yeo, K.-T. Chang, H.-J. Lee, Biomed. Res. Int. 2014, 297241.
- 24J.-Y. Kim, J. Park, J.-Y. Chang, S.-H. Kim, J.-E. Lee, Exp. Neurobiol. 2016, 25, 241–251.
- 25J.-E. Anttila, K.-W. Whitaker, E.-S. Wires, B.-K. Harvey, M. Airavaara, Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 3–14.
- 26
- 26aJ. C. Alves-Filho, E. M. Pålsson-Mcdermott, Front. Immunol. 2016, 7, 145;
- 26bZ. Li, P. Yang, Z. Li, Biochim. Biophys. Acta Rev. Cancer 2014, 1846, 285–296.
- 27
- 27aR. Jafari, H. Almqvist, H. Axelsson, M. Ignatushchenko, T. Lundbäck, P. Nordlund, D. M. Molina, Nat. Protoc. 2014, 9, 2100–2122;
- 27bA. J. Jensen, D. Martinez Molina, T. Lundbäck, Future Med. Chem. 2015, 7, 975–978.
- 28
- 28aS. Wang, Y. Dong, X. Liang, Biosens. Bioelectron. 2018, 109, 1–7;
- 28bA. Olaru, C. Bala, N. Jaffrezic-Renault, H. Y. Aboul-Enein, Crit. Rev. Anal. Chem. 2015, 45, 97–105.
- 29
- 29aW.-J. Zhong, H.-H. Yang, X.-X. Guan, J.-B. Xiong, C.-C. Sun, C.-Y. Zhang, X.-Q. Luo, Y. Zhang, J. Zhang, J.-X. Duan, Y. Zhou, C.-X. Guan, J. Cell. Physiol. 2019, 234, 4641–4654;
- 29bM. Xie, Y. Yu, R. Kang, S. Zhu, L. Yang, L. Zeng, X. Sun, M. Yang, T. R. Billiar, H. Wang, L. Cao, J. Jiang, D. Tang, Nat. Commun. 2016, 7, 13280.
- 30
- 30aJ. Geng, Y. Zhang, S. Li, S. Li, J. Wang, H. Wang, J. Aa, G. Wang, J. Proteome Res. 2019, 18, 57–68;
- 30bL. Yang, M. Xie, M. Yang, Y. Yu, S. Zhu, W. Hou, R. Kang, M.-T. Lotze, T. R. Billiar, H. Wang, L. Cao, D. Tang, Nat. Commun. 2014, 5, 4436;
- 30cE. M. Palsson-Mcdermott, A. M. Curtis, G. Goel, M. A. Lauterbach, F. J. Sheedy, L. E. Gleeson, M. W. van den Bosch, S. R. Quinn, R. Domongo-Fernandez, D. G. Johnston, J. K. Jiang, W. J. Israelsen, J. Keane, C. Thomas, C. Clish, M. Vander Heiden, R. J. Xavier, L. A. O'Neill, Cell Metab. 2015, 21, 65–80.