Scaled-Up Synthesis of Amorphous NiFeMo Oxides and Their Rapid Surface Reconstruction for Superior Oxygen Evolution Catalysis
Dr. Yu Duan
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, CAS Center for Excellence in Nanoscience, Hefei Science Center of CAS, Collaborative Innovation Center of Suzhou Nano Science and Technology, Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Zi-You Yu
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, CAS Center for Excellence in Nanoscience, Hefei Science Center of CAS, Collaborative Innovation Center of Suzhou Nano Science and Technology, Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Shao-Jin Hu
Division of Theoretical and Computational Sciences, Hefei National Laboratory for Physical Sciences at Microscale, CAS Centre for Excellence and Synergetic Innovation Centre in Quantum Information and Quantum Physics, University of Science and Technology of China, Hefei, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Xu-Sheng Zheng
National Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei, 230029 China
Search for more papers by this authorChu-Tian Zhang
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, CAS Center for Excellence in Nanoscience, Hefei Science Center of CAS, Collaborative Innovation Center of Suzhou Nano Science and Technology, Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Hong-He Ding
National Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei, 230029 China
Search for more papers by this authorDr. Bi-Cheng Hu
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, CAS Center for Excellence in Nanoscience, Hefei Science Center of CAS, Collaborative Innovation Center of Suzhou Nano Science and Technology, Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Qi-Qi Fu
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, CAS Center for Excellence in Nanoscience, Hefei Science Center of CAS, Collaborative Innovation Center of Suzhou Nano Science and Technology, Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Zhi-Long Yu
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, CAS Center for Excellence in Nanoscience, Hefei Science Center of CAS, Collaborative Innovation Center of Suzhou Nano Science and Technology, Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorProf. Xiao Zheng
Division of Theoretical and Computational Sciences, Hefei National Laboratory for Physical Sciences at Microscale, CAS Centre for Excellence and Synergetic Innovation Centre in Quantum Information and Quantum Physics, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorProf. Jun-Fa Zhu
National Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei, 230029 China
Search for more papers by this authorCorresponding Author
Prof. Min-Rui Gao
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, CAS Center for Excellence in Nanoscience, Hefei Science Center of CAS, Collaborative Innovation Center of Suzhou Nano Science and Technology, Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorCorresponding Author
Prof. Shu-Hong Yu
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, CAS Center for Excellence in Nanoscience, Hefei Science Center of CAS, Collaborative Innovation Center of Suzhou Nano Science and Technology, Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
Dalian National Laboratory for Clean Energy, Dalian, 116023 China
Search for more papers by this authorDr. Yu Duan
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, CAS Center for Excellence in Nanoscience, Hefei Science Center of CAS, Collaborative Innovation Center of Suzhou Nano Science and Technology, Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Zi-You Yu
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, CAS Center for Excellence in Nanoscience, Hefei Science Center of CAS, Collaborative Innovation Center of Suzhou Nano Science and Technology, Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Shao-Jin Hu
Division of Theoretical and Computational Sciences, Hefei National Laboratory for Physical Sciences at Microscale, CAS Centre for Excellence and Synergetic Innovation Centre in Quantum Information and Quantum Physics, University of Science and Technology of China, Hefei, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorDr. Xu-Sheng Zheng
National Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei, 230029 China
Search for more papers by this authorChu-Tian Zhang
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, CAS Center for Excellence in Nanoscience, Hefei Science Center of CAS, Collaborative Innovation Center of Suzhou Nano Science and Technology, Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Hong-He Ding
National Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei, 230029 China
Search for more papers by this authorDr. Bi-Cheng Hu
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, CAS Center for Excellence in Nanoscience, Hefei Science Center of CAS, Collaborative Innovation Center of Suzhou Nano Science and Technology, Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Qi-Qi Fu
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, CAS Center for Excellence in Nanoscience, Hefei Science Center of CAS, Collaborative Innovation Center of Suzhou Nano Science and Technology, Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorDr. Zhi-Long Yu
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, CAS Center for Excellence in Nanoscience, Hefei Science Center of CAS, Collaborative Innovation Center of Suzhou Nano Science and Technology, Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorProf. Xiao Zheng
Division of Theoretical and Computational Sciences, Hefei National Laboratory for Physical Sciences at Microscale, CAS Centre for Excellence and Synergetic Innovation Centre in Quantum Information and Quantum Physics, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorProf. Jun-Fa Zhu
National Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei, 230029 China
Search for more papers by this authorCorresponding Author
Prof. Min-Rui Gao
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, CAS Center for Excellence in Nanoscience, Hefei Science Center of CAS, Collaborative Innovation Center of Suzhou Nano Science and Technology, Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorCorresponding Author
Prof. Shu-Hong Yu
Division of Nanomaterials & Chemistry, Hefei National Laboratory for Physical Sciences at the Microscale, CAS Center for Excellence in Nanoscience, Hefei Science Center of CAS, Collaborative Innovation Center of Suzhou Nano Science and Technology, Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
Dalian National Laboratory for Clean Energy, Dalian, 116023 China
Search for more papers by this authorGraphical Abstract
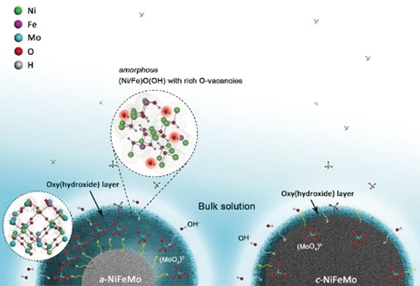
Amorphous NiFeMo oxide (up to 515 g one batch) with homogeneous elemental distribution was synthesized through a facile supersaturated co-precipitation method. The amorphous NiFeMo oxide undergoes rapid surface self-reconstruction during OER that forms a metal oxy(hydroxide) active layer with oxygen vacancies, enabling efficient OER catalysis.
Abstract
The anode oxygen evolution reaction (OER) is known to largely limit the efficiency of electrolyzers owing to its sluggish kinetics. While crystalline metal oxides are promising as OER catalysts, their amorphous phases also show high activities. Efforts to produce amorphous metal oxides have progressed slowly, and how an amorphous structure benefits the catalytic performances remains elusive. Now the first scalable synthesis of amorphous NiFeMo oxide (up to 515 g in one batch) is presented with homogeneous elemental distribution via a facile supersaturated co-precipitation method. In contrast to its crystalline counterpart, amorphous NiFeMo oxide undergoes a faster surface self-reconstruction process during OER, forming a metal oxy(hydroxide) active layer with rich oxygen vacancies, leading to superior OER activity (280 mV overpotential at 10 mA cm−2 in 0.1 m KOH). This opens up the potential of fast, facile, and scale-up production of amorphous metal oxides for high-performance OER catalysts.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201909939-sup-0001-misc_information.pdf13.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD.-Y. Kuo, J. K. Kawasaki, J. N. Nelson, J. Kloppenburg, G. Hautier, K. M. Shen, D. G. Schlom, J. Suntivich, J. Am. Chem. Soc. 2017, 139, 3473–3479;
- 1bF. Li, D. M. Tang, T. Zhang, K. Liao, P. He, D. Golberg, A. Yamada, H. Zhou, Adv. Energy Mater. 2015, 5, 1500294.
- 2S. Zhou, X. Miao, X. Zhao, C. Ma, Y. Qiu, Z. Hu, J. Zhao, L. Shi, J. Zeng, Nat. Commun. 2016, 7, 11510.
- 3K. Fan, H. Chen, Y. Ji, H. Huang, P. M. Claesson, Q. Daniel, B. Philippe, H. Rensmo, F. Li, Y. Luo, L. Sun, Nat. Commun. 2016, 7, 11981.
- 4W. Wang, L. Kuai, W. Cao, M. Huttula, S. Ollikkala, T. Ahopelto, A. P. Honkanen, S. Huotari, M. Yu, B. Geng, Angew. Chem. Int. Ed. 2017, 56, 14977–14981; Angew. Chem. 2017, 129, 15173–15177.
- 5
- 5aL. Yang, Z. Guo, J. Huang, Y. Xi, R. Gao, G. Su, W. Wang, L. Cao, B. Dong, Adv. Mater. 2017, 29, 1704574;
- 5bW. Liu, H. Liu, L. Dang, H. Zhang, X. Wu, B. Yang, Z. Li, X. Zhang, L. Lei, S. Jin, Adv. Funct. Mater. 2017, 27, 1603904;
- 5cR. D. L. Smith, M. S. Prévot, R. D. Fagan, Z. Zhang, P. A. Sedach, M. K. J. Siu, S. Trudel, C. P. Berlinguette, Science 2013, 340, 60–63;
- 5dA. Indra, P. W. Menezes, N. R. Sahraie, A. Bergmann, C. Das, M. Tallarida, D. Schmeißer, P. Strasser, M. Driess, J. Am. Chem. Soc. 2014, 136, 17530–17536;
- 5eG. Chen, W. Zhou, D. Guan, J. Sunarso, Y. Zhu, X. Hu, W. Zhang, Z. Shao, Sci. Adv. 2017, 3, e 1603206;
- 5fB. Zhang, X. Zheng, O. Voznyy, R. Comin, M. Bajdich, M. García-Melchor, L. Han, J. Xu, M. Liu, L. Zheng, F. P. García de Arquer, C. T. Dinh, F. Fan, M. Yuan, E. Yassitepe, N. Chen, T. Regier, P. Liu, Y. Li, P. De Luna, A. Janmohamed, H. L. Xin, H. Yang, A. Vojvodic, E. H. Sargent, Science 2016, 352, 333–337.
- 6
- 6aM. W. Kanan, D. G. Nocera, Science 2008, 321, 1072–1075;
- 6bD. K. Bediako, B. Lassalle-Kaiser, Y. Surendranath, J. Yano, V. K. Yachandra, D. G. Nocera, J. Am. Chem. Soc. 2012, 134, 6801–6809.
- 7
- 7aM. S. Burke, M. G. Kast, L. Trotochaud, A. M. Smith, S. W. Boettcher, J. Am. Chem. Soc. 2015, 137, 3638–3648;
- 7bL. Trotochaud, S. L. Young, J. K. Ranney, S. W. Boettcher, J. Am. Chem. Soc. 2014, 136, 6744–6753;
- 7cM. B. Stevens, C. D. M. Trang, L. J. Enman, J. Deng, S. W. Boettcher, J. Am. Chem. Soc. 2017, 139, 11361–11364.
- 8
- 8aM. W. Louie, A. T. Bell, J. Am. Chem. Soc. 2013, 135, 12329–12337;
- 8bJ. S. Kim, B. Kim, H. Kim, K. Kang, Adv. Energy Mater. 2018, 8, 1702774.
- 9H.-S. Kim, J. B. Cook, H. Lin, J. S. Ko, S. H. Tolbert, V. Ozolins, B. Dunn, Nat. Mater. 2017, 16, 454.
- 10
- 10aY. Wang, Y. Zhang, Z. Liu, C. Xie, S. Feng, D. Liu, M. Shao, S. Wang, Angew. Chem. Int. Ed. 2017, 56, 5867–5871; Angew. Chem. 2017, 129, 5961–5965;
- 10bH. Li, F. Qin, Z. Yang, X. Cui, J. Wang, L. Zhang, J. Am. Chem. Soc. 2017, 139, 3513–3521.
- 11L. Xu, Q. Jiang, Z. Xiao, X. Li, J. Huo, S. Wang, L. Dai, Angew. Chem. Int. Ed. 2016, 55, 5277–5281; Angew. Chem. 2016, 128, 5363–5367.
- 12S. Trasatti, Electrodes of conductive metallic oxides, Vol. 2, Elsevier Scientific Software, 1980.
- 13J. O. Bockris, T. Otagawa, J. Phys. Chem. 1983, 87, 2960–2971.
- 14B. W. Veal, S. K. Kim, P. Zapol, H. Iddir, P. M. Baldo, J. A. Eastman, Nat. Commun. 2016, 7, 11892.
- 15J. Bao, X. Zhang, B. Fan, J. Zhang, M. Zhou, W. Yang, X. Hu, H. Wang, B. Pan, Y. Xie, Angew. Chem. Int. Ed. 2015, 54, 7399–7404; Angew. Chem. 2015, 127, 7507–7512.
- 16A. Pathak, P. Pramanik, Proc. Indian Natl. Sci. Acad. Part A 2001, 67, 47–70.
- 17
- 17aC. Xie, Y. Wang, K. Hu, L. Tao, X. Huang, J. Huo, S. Wang, J. Mater. Chem. A 2017, 5, 87–91;
- 17bY. Y. Chen, Y. Zhang, X. Zhang, T. Tang, H. Luo, S. Niu, Z. H. Dai, L. J. Wan, J. S. Hu, Adv. Mater. 2017, 29, 1703311.
- 18
- 18aE. Fabbri, M. Nachtegaal, T. Binninger, X. Cheng, B.-J. Kim, J. Durst, F. Bozza, T. Graule, R. Schäublin, L. Wiles, M. Pertoso, N. Danilovic, K. E. Ayers, T. J. Schmidt, Nat. Mater. 2017, 16, 925–931;
- 18bA. Bergmann, E. Martinez-Moreno, D. Teschner, P. Chernev, M. Gliech, J. F. De Araújo, T. Reier, H. Dau, P. Strasser, Nat. Commun. 2015, 6, 8625.
- 19Y. R. Zheng, P. Wu, M. R. Gao, X. L. Zhang, F. Y. Gao, H. X. Ju, R. Wu, Q. Gao, R. You, W. X. Huang, S. J. Liu, S. W. Hu, J. Zhu, Z. Li, S. H. Yu, Nat. Commun. 2018, 9, 2533.
- 20K. Banger, Y. Yamashita, K. Mori, R. Peterson, T. Leedham, J. Rickard, H. Sirringhaus, Nat. Mater. 2011, 10, 45–50.
- 21
- 21aM. Ruckman, J. Chen, S. Qiu, P. Kuiper, M. Strongin, B. Dunlap, Phys. Rev. Lett. 1991, 67, 2533;
- 21bM. Imamura, N. Matsubayashi, H. Shimada, J. Phys. Chem. B 2000, 104, 7348–7353.
- 22
- 22aS. Chen, H. Wang, Z. Kang, S. Jin, X. Zhang, X. Zheng, Z. Qi, J. Zhu, B. Pan, Y. Xie, Nat. Commun. 2019, 10, 788;
- 22bH. Liu, A. I. Kozlov, A. P. Kozlova, T. Shido, K. Asakura, Y. Iwasawa, J. Catal. 1999, 185, 252–264.
- 23J. Yang, C. Wang, H. Ju, Y. Sun, S. Xing, J. Zhu, Q. Yang, Adv. Funct. Mater. 2017, 27, 1703864.
- 24
- 24aJ. Yin, Y. Li, F. Lv, M. Lu, K. Sun, W. Wang, L. Wang, F. Cheng, Y. Li, P. Xi, S. Guo, Adv. Mater. 2017, 29, 1704681;
- 24bL. Zhuang, L. Ge, Y. Yang, M. Li, Y. Jia, X. Yao, Z. Zhu, Adv. Mater. 2017, 29, 1606793;
- 24cT. Ling, D. Y. Yan, Y. Jiao, H. Wang, Y. Zheng, X. Zheng, J. Mao, X. W. Du, Z. Hu, M. Jaroniec, S. Z. Qiao, Nat. Commun. 2016, 7, 12876.
- 25C. Gu, S. Hu, X. Zheng, M.-R. Gao, Y.-R. Zheng, L. Shi, Q. Gao, X. Zheng, W. Chu, H.-B. Yao, J. Zhu, S.-H. Yu, Angew. Chem. Int. Ed. 2018, 57, 4020–4024; Angew. Chem. 2018, 130, 4084–4088.
- 26S. Jin, ACS Energy Lett. 2017, 2, 1937–1938.
- 27D. Liu, W. W. Lei, J. Hao, D. D. Liu, B. B. Liu, X. Wang, X. H. Chen, Q. L. Cui, G. T. Zou, J. Liu, S. Jiang, J. Appl. Phys. 2009, 105, 023513.