Arene-Free Ruthenium(II/IV)-Catalyzed Bifurcated Arylation for Oxidative C−H/C−H Functionalizations
Torben Rogge
Institut für Organische und Biomolekulare Chemie, Georg-August-Universität Göttingen, Tammannstraße 2, 37077 Göttingen, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Lutz Ackermann
Institut für Organische und Biomolekulare Chemie, Georg-August-Universität Göttingen, Tammannstraße 2, 37077 Göttingen, Germany
Search for more papers by this authorTorben Rogge
Institut für Organische und Biomolekulare Chemie, Georg-August-Universität Göttingen, Tammannstraße 2, 37077 Göttingen, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Lutz Ackermann
Institut für Organische und Biomolekulare Chemie, Georg-August-Universität Göttingen, Tammannstraße 2, 37077 Göttingen, Germany
Search for more papers by this authorGraphical Abstract
Abstract
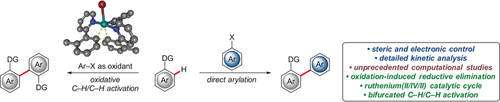
Experimental and computational studies provide detailed insight into the selectivity- and reactivity-controlling factors in bifurcated ruthenium-catalyzed direct C−H arylations and dehydrogenative C−H/C−H functionalizations. Thorough investigations revealed the importance of arene-ligand-free complexes for the formation of biscyclometalated intermediates within a ruthenium(II/IV/II) mechanistic manifold.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201909457-sup-0001-misc_information.pdf5.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For recent reviews, see:
- 1aS. M. Khake, N. Chatani, Trends Chem. 2019, 1, 524–539;
- 1bP. Gandeepan, T. Müller, D. Zell, G. Cera, S. Warratz, L. Ackermann, Chem. Rev. 2019, 119, 2192–2452;
- 1cP. H. Dixneuf, J. F. Soule, Chem. Rev. 2018, 118, 7532–7585;
- 1dP. Gandeepan, L. Ackermann, Chem 2018, 4, 199–222;
- 1eC. S. Wang, J. C. K. Chu, T. Rovis, Angew. Chem. Int. Ed. 2018, 57, 62–101; Angew. Chem. 2018, 130, 64–105;
- 1fK. Murakami, S. Yamada, T. Kaneda, K. Itami, Chem. Rev. 2017, 117, 9302–9332;
- 1gY. Park, Y. Kim, S. Chang, Chem. Rev. 2017, 117, 9247–9301;
- 1hJ. He, M. Wasa, K. S. L. Chan, Q. Shao, J.-Q. Yu, Chem. Rev. 2017, 117, 8754–8786;
- 1iJ. F. Hartwig, M. A. Larsen, ACS Cent. Sci. 2016, 2, 281–292;
- 1jL. McMurray, F. O'Hara, M. J. Gaunt, Chem. Soc. Rev. 2011, 40, 1885–1898, and references therein.
- 2
- 2aT. Cernak, K. D. Dykstra, S. Tyagarajan, P. Vachal, S. W. Krska, Chem. Soc. Rev. 2016, 45, 546–576;
- 2bM. Seki, Org. Process Res. Dev. 2016, 20, 867–877;
- 2cL. Ackermann, Org. Process Res. Dev. 2015, 19, 260–269.
- 3
- 3aT. Bura, J. T. Blaskovits, M. Leclerc, J. Am. Chem. Soc. 2016, 138, 10056–10071;
- 3bD. J. Schipper, K. Fagnou, Chem. Mater. 2011, 23, 1594–1600.
- 4J. Hubrich, T. Himmler, L. Rodefeld, L. Ackermann, ACS Catal. 2015, 5, 4089–4093.
- 5For selected reviews, see:
- 5aP. Nareddy, F. Jordan, M. Szostak, ACS Catal. 2017, 7, 5721–5745;
- 5bJ. A. Leitch, C. G. Frost, Chem. Soc. Rev. 2017, 46, 7145–7153;
- 5cC. Bruneau, P. H. Dixneuf, Top. Organomet. Chem. 2015, 55, 137–188;
- 5dC. Bruneau, Top. Organomet. Chem. 2014, 48, 195–236;
- 5eP. B. Arockiam, C. Bruneau, P. H. Dixneuf, Chem. Rev. 2012, 112, 5879–5918;
- 5fN. Chatani, S. Inoue, K. Yokota, H. Tatamidani, Y. Fukumoto, Pure Appl. Chem. 2010, 82, 1443–1451;
- 5gL. Ackermann, R. Vicente, Top. Curr. Chem. 2010, 292, 211–229;
- 5hL. Ackermann, R. Vicente, A. R. Kapdi, Angew. Chem. Int. Ed. 2009, 48, 9792–9826; Angew. Chem. 2009, 121, 9976–10011.
- 6On July 1, 2019, the prices of platinum, rhodium, iridium, palladium, and ruthenium were 837, 3350, 1480, 1549, and 253 US$ per troy oz, respectively. See: http://www.platinum.matthey.com/prices/price-charts.
- 7B. Li, P. H. Dixneuf, Chem. Soc. Rev. 2013, 42, 5744–5767.
- 8
- 8aS. Oi, E. Aizawa, Y. Ogino, Y. Inoue, J. Org. Chem. 2005, 70, 3113–3119;
- 8bS. Oi, S. Fukita, N. Hirata, N. Watanuki, S. Miyano, Y. Inoue, Org. Lett. 2001, 3, 2579–2581.
- 9
- 9aB. Li, C. Darcel, P. H. Dixneuf, ChemCatChem 2014, 6, 127–130;
- 9bB. Li, C. B. Bheeter, C. Darcel, P. Dixneuf, Top. Catal. 2014, 57, 833–842;
- 9cK. S. Singh, P. H. Dixneuf, ChemCatChem 2013, 5, 1313–1316;
- 9dE. Ferrer Flegeau, C. Bruneau, P. H. Dixneuf, A. Jutand, J. Am. Chem. Soc. 2011, 133, 10161–10170;
- 9eB. Li, C. B. Bheeter, C. Darcel, P. H. Dixneuf, ACS Catal. 2011, 1, 1221–1224;
- 9fP. B. Arockiam, C. Fischmeister, C. Bruneau, P. H. Dixneuf, Angew. Chem. Int. Ed. 2010, 49, 6629–6632; Angew. Chem. 2010, 122, 6779–6782;
- 9gI. Özdemir, S. Demir, B. Çetinkaya, C. Gourlaouen, F. Maseras, C. Bruneau, P. H. Dixneuf, J. Am. Chem. Soc. 2008, 130, 1156–1157.
- 10
- 10aS. R. Yetra, T. Rogge, S. Warratz, J. Struwe, W. Peng, P. Vana, L. Ackermann, Angew. Chem. Int. Ed. 2019, 58, 7490–7494; Angew. Chem. 2019, 131, 7569–7573;
- 10bR. Mei, C. Zhu, L. Ackermann, Chem. Commun. 2016, 52, 13171–13174;
- 10cL. Ackermann, A. V. Lygin, Org. Lett. 2011, 13, 3332–3335;
- 10dL. Ackermann, R. Vicente, H. K. Potukuchi, V. Pirovano, Org. Lett. 2010, 12, 5032–5035;
- 10eL. Ackermann, R. Born, P. Álvarez-Bercedo, Angew. Chem. Int. Ed. 2007, 46, 6364–6367; Angew. Chem. 2007, 119, 6482–6485;
- 10fL. Ackermann, A. Althammer, R. Born, Angew. Chem. Int. Ed. 2006, 45, 2619–2622; Angew. Chem. 2006, 118, 2681–2685;
- 10gL. Ackermann, Org. Lett. 2005, 7, 3123–3125.
- 11
- 11aY. Koseki, K. Kitazawa, M. Miyake, T. Kochi, F. Kakiuchi, J. Org. Chem. 2017, 82, 6503–6510;
- 11bC. J. Teskey, S. M. A. Sohel, D. L. Bunting, S. G. Modha, M. F. Greaney, Angew. Chem. Int. Ed. 2017, 56, 5263–5266; Angew. Chem. 2017, 129, 5347–5350;
- 11cA. Biafora, T. Krause, D. Hackenberger, F. Belitz, L. J. Gooßen, Angew. Chem. Int. Ed. 2016, 55, 14752–14755; Angew. Chem. 2016, 128, 14972–14975;
- 11dL. Huang, D. J. Weix, Org. Lett. 2016, 18, 5432–5435;
- 11eR. K. Chinnagolla, M. Jeganmohan, Chem. Commun. 2014, 50, 2442–2444;
- 11fY. Aihara, N. Chatani, Chem. Sci. 2013, 4, 664–670;
- 11gN. Dastbaravardeh, M. Schnürch, M. D. Mihovilovic, Org. Lett. 2012, 14, 1930–1933;
- 11hF. Kakiuchi, S. Kan, K. Igi, N. Chatani, S. Murai, J. Am. Chem. Soc. 2003, 125, 1698–1699.
- 12For a review, see:
- 12aC.-J. Li, Acc. Chem. Res. 2009, 42, 335–344; for selected examples, see:
- 12bS. Dana, D. Chowdhury, A. Mandal, F. A. S. Chipem, M. Baidya, ACS Catal. 2018, 8, 10173–10179;
- 12cD. Srimani, Y. Ben-David, D. Milstein, Angew. Chem. Int. Ed. 2013, 52, 4012–4015; Angew. Chem. 2013, 125, 4104–4107;
- 12dJ. Dong, Z. Long, F. Song, N. Wu, Q. Guo, J. Lan, J. You, Angew. Chem. Int. Ed. 2013, 52, 580–584; Angew. Chem. 2013, 125, 608–612;
- 12eX. Guo, G. Deng, C.-J. Li, Adv. Synth. Catal. 2009, 351, 2071–2074;
- 12fS. Oi, H. Sato, S. Sugawara, Y. Inoue, Org. Lett. 2008, 10, 1823–1826;
- 12gG. Deng, L. Zhao, C.-J. Li, Angew. Chem. Int. Ed. 2008, 47, 6278–6282; Angew. Chem. 2008, 120, 6374–6378.
- 13L. Ackermann, P. Novák, R. Vicente, V. Pirovano, H. K. Potukuchi, Synthesis 2010, 2245–2253.
- 14L. Ackermann, Acc. Chem. Res. 2014, 47, 281–295.
- 15
- 15aD. L. Davies, S. A. Macgregor, C. L. McMullin, Chem. Rev. 2017, 117, 8649–8709;
- 15bD. Balcells, E. Clot, O. Eisenstein, Chem. Rev. 2010, 110, 749–823.
- 16L. Ackermann, Chem. Rev. 2011, 111, 1315–1345.
- 17CCDC 1942734 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 18
- 18aM. Simonetti, D. M. Cannas, X. Just-Baringo, I. J. Vitorica-Yrezabal, I. Larrosa, Nat. Chem. 2018, 10, 724–731;
- 18bJ. McIntyre, I. Mayoral-Soler, P. Salvador, A. Poater, D. J. Nelson, Catal. Sci. Technol. 2018, 8, 3174–3182;
- 18cM. Simonetti, G. J. Perry, X. C. Cambeiro, F. Julia-Hernandez, J. N. Arokianathar, I. Larrosa, J. Am. Chem. Soc. 2016, 138, 3596–3606;
- 18dP. Marcé, A. J. Paterson, M. F. Mahon, C. G. Frost, Catal. Sci. Technol. 2016, 6, 7068–7076;
- 18eH. H. Al Mamari, E. Diers, L. Ackermann, Chem. Eur. J. 2014, 20, 9739–9743;
- 18fL. Ackermann, A. Althammer, R. Born, Tetrahedron 2008, 64, 6115–6124;
- 18gL. Ackermann, A. Althammer, R. Born, Synlett 2007, 2833–2836.
- 19It is noteworthy that a complex kinetic behavior was observed when 2-bromobenzonitrile was employed as a reagent, indicating a distinct change in mechanism (Figure S9).
- 20For details, see the Supporting Information.
- 21
- 21aJ. Kim, K. Shin, S. Jin, D. Kim, S. Chang, J. Am. Chem. Soc. 2019, 141, 4137–4146;
- 21bS. Y. Hong, Y. Park, Y. Hwang, Y. B. Kim, M.-H. Baik, S. Chang, Science 2018, 359, 1016–1021;
- 21cK. Shin, Y. Park, M.-H. Baik, S. Chang, Nat. Chem. 2017, 10, 218–224.
- 22E. Zuidema, P. W. N. M. van Leeuwen, C. Bo, Organometallics 2005, 24, 3703–3710.
- 23
- 23aK. Naksomboon, J. Poater, F. M. Bickelhaupt, M. Á. Fernández-Ibáñez, J. Am. Chem. Soc. 2019, 141, 6719–6725;
- 23bD. Zell, M. Bursch, V. Müller, S. Grimme, L. Ackermann, Angew. Chem. Int. Ed. 2017, 56, 10378–10382; Angew. Chem. 2017, 129, 10514–10518;
- 23cW. Ma, R. Mei, G. Tenti, L. Ackermann, Chem. Eur. J. 2014, 20, 15248–15251.
- 24S. I. Gorelsky, D. Lapointe, K. Fagnou, J. Am. Chem. Soc. 2008, 130, 10848–10849.