Stereoselective Synthesis of Trisubstituted Vinylboronates from Ketone Enolates Triggered by 1,3-Metalate Rearrangement of Lithium Enolates
Dr. Yue Hu
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Suzhou Research Institute of LICP, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, Lanzhou, 730000 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorWei Sun
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Suzhou Research Institute of LICP, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, Lanzhou, 730000 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorTao Zhang
School of Chemistry and Chemical Engineering, Chongqing University, Chongqing, 400030 P. R. China
College of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou, 450001 P. R. China
Search for more papers by this authorNuo Xu
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Suzhou Research Institute of LICP, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, Lanzhou, 730000 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorJianeng Xu
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Suzhou Research Institute of LICP, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, Lanzhou, 730000 P. R. China
Search for more papers by this authorProf. Dr. Yu Lan
School of Chemistry and Chemical Engineering, Chongqing University, Chongqing, 400030 P. R. China
College of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou, 450001 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Chao Liu
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Suzhou Research Institute of LICP, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, Lanzhou, 730000 P. R. China
Search for more papers by this authorDr. Yue Hu
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Suzhou Research Institute of LICP, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, Lanzhou, 730000 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorWei Sun
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Suzhou Research Institute of LICP, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, Lanzhou, 730000 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorTao Zhang
School of Chemistry and Chemical Engineering, Chongqing University, Chongqing, 400030 P. R. China
College of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou, 450001 P. R. China
Search for more papers by this authorNuo Xu
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Suzhou Research Institute of LICP, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, Lanzhou, 730000 P. R. China
University of Chinese Academy of Sciences, Beijing, 100049 P. R. China
Search for more papers by this authorJianeng Xu
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Suzhou Research Institute of LICP, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, Lanzhou, 730000 P. R. China
Search for more papers by this authorProf. Dr. Yu Lan
School of Chemistry and Chemical Engineering, Chongqing University, Chongqing, 400030 P. R. China
College of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou, 450001 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Chao Liu
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Suzhou Research Institute of LICP, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, Lanzhou, 730000 P. R. China
Search for more papers by this authorGraphical Abstract
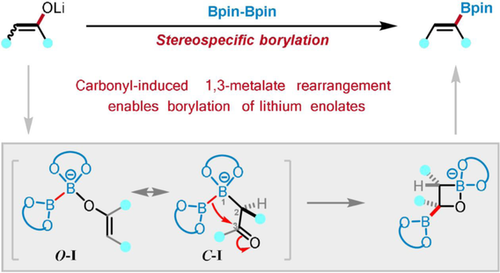
One to three: An unprecedented stereoselective synthesis of trisubstituted vinylboronates from the transition-metal-free borylation of lithium ketone enolates was developed. Carbonyl-induced 1,3-metalate rearrangement through a C-bound boron enolate leads to the stereospecific C−O borylation of lithium enolates. A variety of stereospecific tri- and tetrasubstituted vinylboronates were easily obtained.
Abstract
An unprecedented stereoselective synthesis of trisubstituted vinylboronates is reported to proceed by direct borylation of lithium ketone enolates under transition-metal-free conditions. The stereospecific C−O borylation of lithium enolates was triggered by a carbonyl-induced 1,3-metalate rearrangement via a C-bound boron enolate. DFT calculations and control experiments revealed that the stereoselectivity is controlled by sterics. A variety of stereospecific trisubstituted vinylboronates, together with several tetrasubstituted vinylboronates, were conveniently synthesized with the newly developed methodology. Based on the transformation of stereospecific vinylboronate, a single isomer of Dienestrol was efficiently obtained.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201909235-sup-0001-misc_information.pdf10.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD. G. Hall, Boronic Acids: Preparation and Applications in Organic Synthesis Medicine and Materials, Vols. 1 and 2, 2nd ed., Wiley-VCH, Weinheim, 2011;
10.1002/9783527639328 Google Scholar
- 1bR. S. Dhillon, Hydroboration and Organic Synthesis, Springer, Berlin, 2007.
- 2R. J. Armstrong, V. K. Aggarwal, Synthesis 2017, 49, 3323–3336.
- 3A. J. J. Lennox, G. C. Lloyd-Jones, Chem. Soc. Rev. 2014, 43, 412–443.
- 4N. A. Petasis, I. Akritopoulou, Tetrahedron Lett. 1993, 34, 583–586.
- 5
- 5aL. Zhang, G. J. Lovinger, E. K. Edelstein, A. A. Szymaniak, M. P. Chierchia, J. P. Morken, Science 2016, 351, 70–74;
- 5bM. Kischkewitz, K. Okamoto, C. Mück-Lichtenfeld, A. Studer, Science 2017, 355, 936;
- 5cM. Silvi, C. Sandford, V. K. Aggarwal, J. Am. Chem. Soc. 2017, 139, 5736–5739.
- 6
- 6aJ. Carreras, A. Caballero, P. J. Pérez, Chem. Asian J. 2019, 14, 329–343;
- 6bT.-J. Hu, G. Zhang, Y.-H. Chen, C.-G. Feng, G.-Q. Lin, J. Am. Chem. Soc. 2016, 138, 2897–2900;
- 6cW. Guan, A. K. Michael, M. L. McIntosh, L. Koren-Selfridge, J. P. Scott, T. B. Clark, J. Org. Chem. 2014, 79, 7199–7204;
- 6dW. Su, T.-J. Gong, Q. Zhang, Q. Zhang, B. Xiao, Y. Fu, ACS Catal. 2016, 6, 6417–6421;
- 6eO. Zhurakovskyi, R. M. P. Dias, A. Noble, V. K. Aggarwal, Org. Lett. 2018, 20, 3136–3139.
- 7
- 7aH. R. Kim, J. Yun, Chem. Commun. 2011, 47, 2943–2945;
- 7bB. Sundararaju, A. Fürstner, Angew. Chem. Int. Ed. 2013, 52, 14050–14054; Angew. Chem. 2013, 125, 14300–14304;
- 7cK. Semba, N. Bessho, T. Fujihara, J. Terao, Y. Tsuji, Angew. Chem. Int. Ed. 2014, 53, 9007–9011; Angew. Chem. 2014, 126, 9153–9157;
- 7dK. Semba, M. Shinomiya, T. Fujihara, J. Terao, Y. Tsuji, Chem. Eur. J. 2013, 19, 7125–7132;
- 7eL. Mao, R. Bertermann, K. Emmert, K. J. Szabó, T. B. Marder, Org. Lett. 2017, 19, 6586–6589;
- 7fW. Yuan, X. Zhang, Y. Yu, S. Ma, Chem. Eur. J. 2013, 19, 7193–7202;
- 7gY. D. Bidal, F. Lazreg, C. S. J. Cazin, ACS Catal. 2014, 4, 1564–1569.
- 8
- 8aW. B. Reid, D. A. Watson, Org. Lett. 2018, 20, 6832–6835;
- 8bN. Kirai, S. Iguchi, T. Ito, J. Takaya, N. Iwasawa, Bull. Chem. Soc. Jpn. 2013, 86, 784–799.
- 9J. R. Coombs, L. Zhang, J. P. Morken, Org. Lett. 2015, 17, 1708–1711.
- 10S. P. Thomas, R. M. French, V. Jheengut, V. K. Aggarwal, Chem. Rec. 2009, 9, 24–39.
- 11
- 11aL. Wang, T. Zhang, W. Sun, Z. He, C. Xia, Y. Lan, C. Liu, J. Am. Chem. Soc. 2017, 139, 5257–5264;
- 11bD. Shi, L. Wang, C. Xia, C. Liu, Angew. Chem. Int. Ed. 2018, 57, 10318–10322; Angew. Chem. 2018, 130, 10475–10479;
- 11cZ. He, Y. Hu, C. Xia, C. Liu, Org. Biomol. Chem. 2019, 17, 6099–6113;
- 11dL. Wang, W. Sun, C. Liu, Chin. J. Catal. 2018, 39, 1725–1729.
- 12J. Zabicky, The chemistry of metal enolates, Wiley, Hoboken, 2009.
- 13F. K. Scharnagl, S. K. Bose, T. B. Marder, Org. Biomol. Chem. 2017, 15, 1738–1752.
- 14E. C. Neeve, S. J. Geier, I. A. I. Mkhalid, S. A. Westcott, T. B. Marder, Chem. Rev. 2016, 116, 9091–9161.
- 15
- 15aK. Endo, M. Hirokami, T. Shibata, J. Org. Chem. 2010, 75, 3469–3472;
- 15bS. H. Cho, J. F. Hartwig, Chem. Sci. 2014, 5, 694–698;
- 15cE. La Cascia, A. B. Cuenca, E. Fernández, Chem. Eur. J. 2016, 22, 18737–18741;
- 15dT. C. Stephens, G. Pattison, Org. Lett. 2017, 19, 3498–3501;
- 15eS. Namirembe, C. Gao, R. P. Wexler, J. P. Morken, Org. Lett. 2019, 21, 4392–4394.
- 16L. M. Jackman, B. C. Lange, J. Am. Chem. Soc. 1981, 103, 4494–4499.
- 17The single E-isomers have been synthesized via Pd-catalyzed selective monoarylation of 1,1-diborylalkenes, see: H. Wen, L. Zhang, S. Zhu, G. Liu, Z. Huang, ACS Catal. 2017, 7, 6419–6425.
- 18H. Li, Y. Zhang, J. Wang, Synthesis 2013, 45, 3090–3098.
- 19W. Sun, L. Wang, C. Xia, C. Liu, Angew. Chem. Int. Ed. 2018, 57, 5501–5505; Angew. Chem. 2018, 130, 5599–5603.
- 20
- 20aR. D. Dewhurst, E. C. Neeve, H. Braunschweig, T. B. Marder, Chem. Commun. 2015, 51, 9594–9607;
- 20bS. Pietsch, E. C. Neeve, D. C. Apperley, R. Bertermann, F. Mo, D. Qiu, M. S. Cheung, L. Dang, J. Wang, U. Radius, Z. Lin, C. Kleeberg, T. B. Marder, Chem. Eur. J. 2015, 21, 7082–7098.
- 21
- 21aZ. He, A. Zajdlik, A. K. Yudin, Dalton Trans. 2014, 43, 11434–11451;
- 21bQ.-Q. Cheng, S.-F. Zhu, Y.-Z. Zhang, X.-L. Xie, Q.-L. Zhou, J. Am. Chem. Soc. 2013, 135, 14094–14097;
- 21cD. Chen, X. Zhang, W.-Y. Qi, B. Xu, M.-H. Xu, J. Am. Chem. Soc. 2015, 137, 5268–5271;
- 21dJ.-M. Yang, Y.-T. Zhao, Z.-Q. Li, X.-S. Gu, S.-F. Zhu, Q.-L. Zhou, ACS Catal. 2018, 8, 7351–7355;
- 21eS.-C. Ren, F.-L. Zhang, A.-Q. Xu, Y. Yang, M. Zheng, X. Zhou, Y. Fu, Y.-F. Wang, Nat. Commun. 2019, 10, 1934.
- 22The direct transformation of O-bound boron enolate by boryl metathesis process has also been considered, and it is energetically unfavorable (see details in the Supporting Information).
- 23J. Liu, P. Chakraborty, H. Zhang, L. Zhong, Z.-X. Wang, X. Huang, ACS Catal. 2019, 9, 2610–2617.
- 24R. J. Armstrong, C. Garcia-Ruiz, E. L. Myers, V. K. Aggarwal, Angew. Chem. Int. Ed. 2017, 56, 786–790; Angew. Chem. 2017, 129, 804–808.
- 25X. T. Li, Q. S. Gu, X. Y. Dong, X. Meng, X. Y. Liu, Angew. Chem. Int. Ed. 2018, 57, 7668–7672; Angew. Chem. 2018, 130, 7794–7798.
- 26A. Noble, S. Roesner, V. K. Aggarwal, Angew. Chem. Int. Ed. 2016, 55, 15920–15924; Angew. Chem. 2016, 128, 16152–16156.