Access to Multifunctionalized Benzofurans by Aryl Nickelation of Alkynes: Efficient Synthesis of the Anti-Arrhythmic Drug Amiodarone
Dr. Naeem Iqbal
Department of Chemistry, Chung-Ang University, 84 Heukseok-ro, Dongjak-gu, Seoul, 06974 Republic of Korea
Search for more papers by this authorNaila Iqbal
Department of Chemistry, Chung-Ang University, 84 Heukseok-ro, Dongjak-gu, Seoul, 06974 Republic of Korea
Search for more papers by this authorCorresponding Author
Prof. Dr. Debabrata Maiti
Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai, 400076 India
Search for more papers by this authorCorresponding Author
Prof. Dr. Eun Jin Cho
Department of Chemistry, Chung-Ang University, 84 Heukseok-ro, Dongjak-gu, Seoul, 06974 Republic of Korea
Search for more papers by this authorDr. Naeem Iqbal
Department of Chemistry, Chung-Ang University, 84 Heukseok-ro, Dongjak-gu, Seoul, 06974 Republic of Korea
Search for more papers by this authorNaila Iqbal
Department of Chemistry, Chung-Ang University, 84 Heukseok-ro, Dongjak-gu, Seoul, 06974 Republic of Korea
Search for more papers by this authorCorresponding Author
Prof. Dr. Debabrata Maiti
Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai, 400076 India
Search for more papers by this authorCorresponding Author
Prof. Dr. Eun Jin Cho
Department of Chemistry, Chung-Ang University, 84 Heukseok-ro, Dongjak-gu, Seoul, 06974 Republic of Korea
Search for more papers by this authorGraphical Abstract
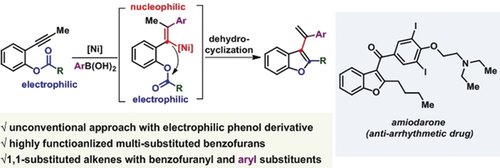
An unconventional nickel-catalyzed reaction has been developed for the synthesis of multifunctionalized benzofurans through the aryl nickelation of alkynes. A nucleophilic vinyl NiII species, from regioselective syn-aryl nickelation of an alkyne, undergoes an intramolecular cyclization with phenol ester to yield highly functionalized 1,1-disubstituted alkenes with 3-benzofuranyl and (hetero)aryl substituents.
Abstract
An unconventional nickel-catalyzed reaction was developed for the synthesis of multifunctionalized benzofurans from alkyne-tethered phenolic esters. The transformation involves the generation of a nucleophilic vinyl NiII species by the regioselective syn-aryl nickelation of an alkyne, which then undergoes an intramolecular cyclization with phenol ester to yield highly functionalized 1,1-disubstituted alkenes with 3-benzofuranyl and (hetero)aryl substituents. The methodology can be used for the late-stage benzofuran incorporation of various drug molecules and natural products, such as 2-propylvaleric acid, gemfibrozil, biotin, and lithocholic acid. Furthermore, this arylative cyclization method was successfully applied for the efficient synthesis of the anti-arrhythmic drug amiodarone.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201909015-sup-0001-misc_information.pdf12.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For benzofuran-containing natural products, see:
- 1aP. Cagniant, D. Cagniant, in Advances in Heterocyclic Chemistry, Vol. 18, Elsevier, Amsterdam, 1975, pp. 337–482;
10.1016/S0065-2725(08)60132-4 Google Scholar
- 1bM. Inoue, M. W. Carson, A. J. Frontier, S. J. Danishefsky, J. Am. Chem. Soc. 2001, 123, 1878–1889;
- 1cH. Khanam, Eur. J. Med. Chem. 2015, 97, 483–504;
- 1dA. Radadiya, A. Shah, Eur. J. Med. Chem. 2015, 97, 356–376.
- 2For biological properties of benzofurans, see:
- 2aC. J. Hamilton, A. Saravanamuthu, A. H. Fairlamb, I. M. Eggleston, Bioorg. Med. Chem. 2003, 11, 3683–3693;
- 2bK. M. Dawood, H. Abdel-Gawad, E. A. Rageb, M. Ellithey, H. A. Mohamed, Bioorg. Med. Chem. 2006, 14, 3672–3680;
- 2cS. Rizzo, C. L. Rivière, L. Piazzi, A. Bisi, S. Gobbi, M. Bartolini, V. Andrisano, F. Morroni, A. Tarozzi, J.-P. Monti, J. Med. Chem. 2008, 51, 2883–2886.
- 3For selected reviews on the synthesis of benzofurans, see:
- 3aA. R. Katritzky, S. Rachwal, Chem. Rev. 2011, 111, 7063–7120;
- 3bX.-F. Wu, H. Neumann, M. Beller, Chem. Rev. 2013, 113, 1–35;
- 3cS. Agasti, A. Dey, D. Maiti, Chem. Commun. 2017, 53, 6544–6556;
- 3dI. Khan, A. Ibrar, S. A. Shehzadi, Coord. Chem. Rev. 2019, 380, 440–470.
- 4For selected reports on synthesis of benzofurans, see:
- 4aI. Nakamura, Y. Mizushima, Y. Yamamoto, J. Am. Chem. Soc. 2005, 127, 15022–15023;
- 4bK. W. Anderson, T. Ikawa, R. E. Tundel, S. L. Buchwald, J. Am. Chem. Soc. 2006, 128, 10694–10695;
- 4cL. Ackermann, L. T. Kaspar, J. Org. Chem. 2007, 72, 6149–6153;
- 4dX. Guo, R. Yu, H. Li, Z. Li, J. Am. Chem. Soc. 2009, 131, 17387–17393;
- 4eT. J. Maimone, S. L. Buchwald, J. Am. Chem. Soc. 2010, 132, 9990–9991;
- 4fT. Kobatake, D. Fujino, S. Yoshida, H. Yorimitsu, K. Oshima, J. Am. Chem. Soc. 2010, 132, 11838–11840;
- 4gD.-H. Lee, K.-H. Kwon, C. S. Yi, J. Am. Chem. Soc. 2012, 134, 7325–7328;
- 4hN. Ortega, S. Urban, B. Beiring, F. Glorius, Angew. Chem. Int. Ed. 2012, 51, 1710–1713; Angew. Chem. 2012, 124, 1742–1745;
- 4iN. Sakiyama, K. Noguchi, K. Tanaka, Angew. Chem. Int. Ed. 2012, 51, 5976–5980; Angew. Chem. 2012, 124, 6078–6082;
- 4jY. Ookubo, A. Wakamiya, H. Yorimitsu, A. Osuka, Chem. Eur. J. 2012, 18, 12690–12697;
- 4kY. Li, J. Waser, Beilstein J. Org. Chem. 2013, 9, 1763–1767;
- 4lM. R. Kuram, M. Bhanuchandra, A. K. Sahoo, Angew. Chem. Int. Ed. 2013, 52, 4607–4612; Angew. Chem. 2013, 125, 4705–4710;
- 4mR. Zhu, J. Wei, Z. Shi, Chem. Sci. 2013, 4, 3706–3711;
- 4nH. P. L. Gemoets, I. Kalvet, A. V. Nyuchev, N. Erdmann, V. Hessel, F. Schoenebeck, T. Noël, Chem. Sci. 2017, 8, 1046–1055;
- 4oK. Okamoto, M. Hori, T. Yanagi, K. Murakami, K. Nogi, H. Yorimitsu, Angew. Chem. Int. Ed. 2018, 57, 14230–14234; Angew. Chem. 2018, 130, 14426–14430;
- 4pK. Yang, A. P. Pulis, G. J. P. Perry, D. J. Procter, Org. Lett. 2018, 20, 7498–7503;
- 4qS. Samanta, A. Hajra, Org. Biomol. Chem. 2018, 16, 7012–7016;
- 4rS. Samanta, A. Hajra, Chem. Commun. 2018, 54, 3379–3382.
- 5For some reports on the synthesis of benzofurans by our group, see:
- 5aU. Sharma, T. Naveen, A. Maji, S. Manna, D. Maiti, Angew. Chem. Int. Ed. 2013, 52, 12669–12673; Angew. Chem. 2013, 125, 12901–12905;
- 5bS. Agasti, S. Maity, K. J. Szabo, D. Maiti, Adv. Synth. Catal. 2015, 357, 2331–2338;
- 5cS. Agasti, U. Sharma, T. Naveen, D. Maiti, Chem. Commun. 2015, 51, 5375–5378.
- 6For selected reports on the synthesis of benzofurans from 2-alkynyl phenols, see:
- 6aA. Fürstner, P. W. Davies, J. Am. Chem. Soc. 2005, 127, 15024–15025;
- 6bM. Nakamura, L. Ilies, S. Otsubo, E. Nakamura, Angew. Chem. Int. Ed. 2006, 45, 944–947; Angew. Chem. 2006, 118, 958–961;
- 6cM. Nakamura, L. Ilies, S. Otsubo, E. Nakamura, Org. Lett. 2006, 8, 2803–2805;
- 6dA. Boyer, N. Isono, S. Lackner, M. Lautens, Tetrahedron 2010, 66, 6468–6482;
- 6eN. A. Markina, Y. Chen, R. C. Larock, Tetrahedron 2013, 69, 2701–2713;
- 6fZ. Han, L. Zhang, Z. Li, R. Fan, Angew. Chem. Int. Ed. 2014, 53, 6805–6809; Angew. Chem. 2014, 126, 6923–6927;
- 6gJ. J. Hirner, D. J. Faizi, S. A. Blum, J. Am. Chem. Soc. 2014, 136, 4740–4745;
- 6hY. Li, G. Gryn′ova, F. Saenz, X. Jeanbourquin, K. Sivula, C. Corminboeuf, J. Waser, Chem. Eur. J. 2017, 23, 8058–8065;
- 6iT. Namba, Y. Hayashi, S. Kawauchi, Y. Shibata, K. Tanaka, Chem. Eur. J. 2018, 24, 7161–7171;
- 6jW. Yi, W. Chen, F.-X. Liu, Y. Zhong, D. Wu, Z. Zhou, H. Gao, ACS Catal. 2018, 8, 9508–9519.
- 7Methods for 3-alkenyl benzofurans, see:
- 7aC. Martínez, R. Álvarez, J. M. Aurrecoechea, Org. Lett. 2009, 11, 1083–1086;
- 7bR. Álvarez, C. Martínez, Y. Madich, J. G. Denis, J. M. Aurrecoechea, A. R. de Lera, Chem. Eur. J. 2010, 16, 12746–12753.
- 8C. Lei, Y. J. Yip, J. S. Zhou, J. Am. Chem. Soc. 2017, 139, 6086–6089.
- 9Some reviews about Ni catalysis:
- 9aS. Z. Tasker, E. A. Standley, T. F. Jamison, Nature 2014, 509, 299;
- 9bV. P. Ananikov, ACS Catal. 2015, 5, 1964–1971.
- 10
- 10aC. Clarke, C. A. Incerti-Pradillos, H. W. Lam, J. Am. Chem. Soc. 2016, 138, 8068–8071;
- 10bC. Yap, G. M. Lenagh-Snow, S. N. Karad, W. Lewis, L. J. Diorazio, H. W. Lam, Angew. Chem. Int. Ed. 2017, 56, 8216–8220; Angew. Chem. 2017, 129, 8328–8332;
- 10cS. M. Gillbard, C.-H. Chung, S. N. Karad, H. Panchal, W. Lewis, H. W. Lam, Chem. Commun. 2018, 54, 11769–11772;
- 10dS. N. Karad, H. Panchal, C. Clarke, W. Lewis, H. W. Lam, Angew. Chem. Int. Ed. 2018, 57, 9122–9125; Angew. Chem. 2018, 130, 9260–9263.
- 11
- 11aX. You, X. Xie, G. Wang, M. Xiong, R. Sun, H. Chen, Y. Liu, Chem. Eur. J. 2016, 22, 16765–16769;
- 11bX. Zhang, X. Xie, Y. Liu, Chem. Sci. 2016, 7, 5815–5820;
- 11cX. Zhang, X. Xie, Y. Liu, J. Am. Chem. Soc. 2018, 140, 7385–7389.
- 12
- 12aL. Zhou, M. Zhang, W. Li, J. Zhang, Angew. Chem. Int. Ed. 2014, 53, 6542–6545; Angew. Chem. 2014, 126, 6660–6663;
- 12bJ. Sun, J.-K. Qiu, Y.-N. Wu, W.-J. Hao, C. Guo, G. Li, S.-J. Tu, B. Jiang, Org. Lett. 2017, 19, 754–757;
- 12cJ. Wang, X. Cao, S. Lv, C. Zhang, S. Xu, M. Shi, J. Zhang, Nat. Commun. 2017, 8, 14625;
- 12dS. Ge, W. Cao, T. Kang, B. Hu, H. Zhang, Z. Su, X. Liu, X. Feng, Angew. Chem. Int. Ed. 2019, 58, 4017–4021; Angew. Chem. 2019, 131, 4057–4061.
- 13The reaction with Ni(COD)2 might proceed through an oxidative cyclization of Ni0 with alkyne and carbonyl moieties in 1 a. See: M. Murakami, S. Ashida, T. Matsuda, J. Am. Chem. Soc. 2005, 127, 6932–6933.
- 14
- 14aC. Gürtler, S. L. Buchwald, Chem. Eur. J. 1999, 5, 3107–3112;
10.1002/(SICI)1521-3765(19991105)5:11<3107::AID-CHEM3107>3.0.CO;2-# CAS Web of Science® Google Scholar
- 14bH. Tsuji, E. Nakamura, Acc. Chem. Res. 2017, 50, 396–406.
- 15M. U. Ali, D. Fitzpatrick-Lewis, M. Kenny, P. Raina, D. L. Atkins, J. Soar, J. Nolan, G. Ristagno, D. Sherifali, Resuscitation 2018, 132, 63–72.
- 16G.-Z. Wang, X.-L. Li, J.-J. Dai, H.-J. Xu, J. Org. Chem. 2014, 79, 7220–7225.
- 17M. J. Bosiak, ACS Catal. 2016, 6, 2429–2434.
- 18W. Huang, J. Xu, C. Liu, Z. Chen, Y. Gu, J. Org. Chem. 2019, 84, 2941–2950.
- 19B. Bhushan, A. Erdmann, Y. Zhang, R. Belle, C. Johannson, U. Oppermann, R. J. Hopkinson, C. J. Schofield, A. Kawamura, Bioorg. Med. Chem. 2018, 26, 2984–2991.