Molecular Design Strategy for Ordered Mesoporous Stoichiometric Metal Oxide
Dr. Changyao Wang
Laboratory of Advanced Materials, Department of Chemistry, Shanghai Key Lab of Molecular Catalysis and Innovative Materials, iChEM and State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200433 China
Search for more papers by this authorDr. Xiaoyue Wan
Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Jiangsu National Synergetic Innovation Center for Advanced Materials, Nanjing Tech University, Nanjing, 211816 China
Search for more papers by this authorLinlin Duan
Laboratory of Advanced Materials, Department of Chemistry, Shanghai Key Lab of Molecular Catalysis and Innovative Materials, iChEM and State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200433 China
Search for more papers by this authorPeiyuan Zeng
Laboratory of Advanced Materials, Department of Chemistry, Shanghai Key Lab of Molecular Catalysis and Innovative Materials, iChEM and State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200433 China
Search for more papers by this authorDr. Liangliang Liu
Laboratory of Advanced Materials, Department of Chemistry, Shanghai Key Lab of Molecular Catalysis and Innovative Materials, iChEM and State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200433 China
Search for more papers by this authorDingyi Guo
Laboratory of Advanced Materials, Department of Chemistry, Shanghai Key Lab of Molecular Catalysis and Innovative Materials, iChEM and State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200433 China
Search for more papers by this authorDr. Yuan Xia
Laboratory of Advanced Materials, Department of Chemistry, Shanghai Key Lab of Molecular Catalysis and Innovative Materials, iChEM and State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200433 China
Search for more papers by this authorProf. Ahmed A. Elzatahry
Materials Science and Technology Program, College of Arts and Sciences, Qatar University, PO Box 2713, Doha, Qatar
Search for more papers by this authorProf. Yongyao Xia
Laboratory of Advanced Materials, Department of Chemistry, Shanghai Key Lab of Molecular Catalysis and Innovative Materials, iChEM and State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200433 China
Search for more papers by this authorCorresponding Author
Prof. Wei Li
Laboratory of Advanced Materials, Department of Chemistry, Shanghai Key Lab of Molecular Catalysis and Innovative Materials, iChEM and State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200433 China
Search for more papers by this authorCorresponding Author
Prof. Dongyuan Zhao
Laboratory of Advanced Materials, Department of Chemistry, Shanghai Key Lab of Molecular Catalysis and Innovative Materials, iChEM and State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200433 China
Search for more papers by this authorDr. Changyao Wang
Laboratory of Advanced Materials, Department of Chemistry, Shanghai Key Lab of Molecular Catalysis and Innovative Materials, iChEM and State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200433 China
Search for more papers by this authorDr. Xiaoyue Wan
Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Jiangsu National Synergetic Innovation Center for Advanced Materials, Nanjing Tech University, Nanjing, 211816 China
Search for more papers by this authorLinlin Duan
Laboratory of Advanced Materials, Department of Chemistry, Shanghai Key Lab of Molecular Catalysis and Innovative Materials, iChEM and State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200433 China
Search for more papers by this authorPeiyuan Zeng
Laboratory of Advanced Materials, Department of Chemistry, Shanghai Key Lab of Molecular Catalysis and Innovative Materials, iChEM and State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200433 China
Search for more papers by this authorDr. Liangliang Liu
Laboratory of Advanced Materials, Department of Chemistry, Shanghai Key Lab of Molecular Catalysis and Innovative Materials, iChEM and State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200433 China
Search for more papers by this authorDingyi Guo
Laboratory of Advanced Materials, Department of Chemistry, Shanghai Key Lab of Molecular Catalysis and Innovative Materials, iChEM and State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200433 China
Search for more papers by this authorDr. Yuan Xia
Laboratory of Advanced Materials, Department of Chemistry, Shanghai Key Lab of Molecular Catalysis and Innovative Materials, iChEM and State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200433 China
Search for more papers by this authorProf. Ahmed A. Elzatahry
Materials Science and Technology Program, College of Arts and Sciences, Qatar University, PO Box 2713, Doha, Qatar
Search for more papers by this authorProf. Yongyao Xia
Laboratory of Advanced Materials, Department of Chemistry, Shanghai Key Lab of Molecular Catalysis and Innovative Materials, iChEM and State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200433 China
Search for more papers by this authorCorresponding Author
Prof. Wei Li
Laboratory of Advanced Materials, Department of Chemistry, Shanghai Key Lab of Molecular Catalysis and Innovative Materials, iChEM and State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200433 China
Search for more papers by this authorCorresponding Author
Prof. Dongyuan Zhao
Laboratory of Advanced Materials, Department of Chemistry, Shanghai Key Lab of Molecular Catalysis and Innovative Materials, iChEM and State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai, 200433 China
Search for more papers by this authorGraphical Abstract
Abstract
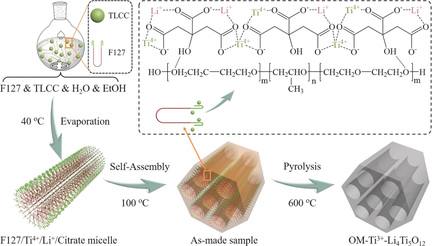
A molecular design strategy is used to construct ordered mesoporous Ti3+-doped Li4Ti5O12 nanocrystal frameworks (OM-Ti3+-Li4Ti5O12) by the stoichiometric cationic coordination assembly process. Ti4+/Li+-citrate chelate is designed as a new molecular precursor, in which the citrate can not only stoichiometrically coordinate Ti4+ with Li+ homogeneously at the atomic scale, but also interact strongly with the PEO segments in the Pluronic F127. These features make the co-assembly and crystallization process more controllable, thus benefiting for the formation of the ordered mesostructures. The resultant OM-Ti3+-Li4Ti5O12 shows excellent rate (143 mAh g−1 at 30 C) and cycling performances (<0.005 % fading per cycle). This work could open a facile avenue to constructing stoichiometric ordered mesoporous oxides or minerals with highly crystalline frameworks.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201907748-sup-0001-misc_information.pdf1.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. N. Zhu, H. J. Liu, J. H. Zhuang, C. X. Wang, Y. G. Wang, Y. Y. Xia, Energy Environ. Sci. 2011, 4, 4016–4022;
- 1bY. Q. Wang, L. Gu, Y.-G. Guo, H. Li, X.-Q. He, S. Tsukimoto, Y. Ikuhara, L.-J. Wan, J. Am. Chem. Soc. 2012, 134, 7874–7879;
- 1cY. Tang, Y. Zhang, W. Li, B. Ma, X. Chen, Chem. Soc. Rev. 2015, 44, 5926–5940;
- 1dY. Tang, Y. Zhang, X. Rui, D. Qi, Y. Luo, W. R. Leow, S. Chen, J. Guo, J. Wei, W. Li, J. Deng, Y. Lai, B. Ma, X. Chen, Adv. Mater. 2016, 28, 1567–1576.
- 2
- 2aX. Lu, L. Gu, Y.-S. Hu, H.-C. Chiu, H. Li, G. P. Demopoulos, L. Chen, J. Am. Chem. Soc. 2015, 137, 1581–1586;
- 2bZ. Chen, I. Belharouak, Y.-K. Sun, K. Amine, Adv. Funct. Mater. 2013, 23, 959–969.
- 3
- 3aH. G. Jung, S. T. Myung, C. S. Yoon, S. B. Son, K. H. Oh, K. Amine, B. Scrosati, Y. K. Sun, Energy Environ. Sci. 2011, 4, 1345–1351;
- 3bY. Wang, E. Hosono, K. Wang, H. Zhou, Angew. Chem. Int. Ed. 2008, 47, 7461–7465; Angew. Chem. 2008, 120, 7571–7575;
- 3cX. Jia, Y. Kan, X. Zhu, G. Ning, Y. Lu, F. Wei, Nano Energy 2014, 10, 344–352;
- 3dK.-S. Park, A. Benayad, D.-J. Kang, S.-G. Doo, J. Am. Chem. Soc. 2008, 130, 14930–14931.
- 4H. Song, S.-W. Yun, H.-H. Chun, M.-G. Kim, K. Y. Chung, H. S. Kim, B.-W. Cho, Y.-T. Kim, Energy Environ. Sci. 2012, 5, 9903–9913.
- 5
- 5aL. Yu, H. B. Wu, X. W. Lou, Adv. Mater. 2013, 25, 2296–2300;
- 5bN. Li, G. Zhou, F. Li, L. Wen, H.-M. Cheng, Adv. Funct. Mater. 2013, 23, 5429–5435;
- 5cS. Chen, Y. Xin, Y. Zhou, Y. Ma, H. Zhou, L. Qi, Energy Environ. Sci. 2014, 7, 1924–1930;
- 5dJ. Liu, K. Song, P. A. van Aken, J. Maier, Y. Yu, Nano Lett. 2014, 14, 2597–2603;
- 5eL. Shen, X. Zhang, E. Uchaker, C. Yuan, G. Cao, Adv. Energy Mater. 2012, 2, 691–698.
- 6
- 6aW. J. H. Borghols, M. Wagemaker, U. Lafont, E. M. Kelder, F. M. Mulder, J. Am. Chem. Soc. 2009, 131, 17786–17792;
- 6bZ. Wang, D. Luan, F. Y. C. Boey, X. W. Lou, J. Am. Chem. Soc. 2011, 133, 4738–4741;
- 6cE. Kang, Y. S. Jung, A. S. Cavanagh, G.-H. Kim, S. M. George, A. C. Dillon, J. K. Kim, J. Lee, Adv. Funct. Mater. 2011, 21, 2430–2438;
- 6dN. Wu, Z.-Z. Yang, H.-R. Yao, Y.-X. Yin, L. Gu, Y.-G. Guo, Angew. Chem. Int. Ed. 2015, 54, 5757–5761; Angew. Chem. 2015, 127, 5849–5853.
- 7
- 7aW. Li, J. Liu, D. Zhao, Nat. Rev. Mater. 2016, 1, 16023;
- 7bH. Liu, D. Su, R. Zhou, B. Sun, G. Wang, S. Z. Qiao, Adv. Energy Mater. 2012, 2, 970–975;
- 7cM. Pramanik, Y. Tsujimoto, V. Malgras, S. X. Dou, J. H. Kim, Y. Yamauchi, Chem. Mater. 2015, 27, 1082–1089;
- 7dC. Wang, Y. Zhao, L. Zhou, Y. Liu, W. Zhang, Z. Zhao, W. N. Hozzein, H. M. S. Alharbi, W. Li, D. Zhao, J. Mater. Chem. A 2018, 6, 21550–21557;
- 7eD. Gu, W. Li, F. Wang, H. Bongard, B. Spliethoff, W. Schmidt, C. Weidenthaler, Y. Xia, D. Zhao, F. Schuth, Angew. Chem. Int. Ed. 2015, 54, 7060–7064; Angew. Chem. 2015, 127, 7166–7170.
- 8
- 8aD. Gu, F. Schuth, Chem. Soc. Rev. 2014, 43, 313–344;
- 8bY. Shi, Y. Wan, D. Zhao, Chem. Soc. Rev. 2011, 40, 3854–3878;
- 8cY. Wan, D. Zhao, Chem. Rev. 2007, 107, 2821–2860.
- 9J. Wang, C. Xue, Y. Lv, F. Zhang, B. Tu, D. Zhao, Carbon 2011, 49, 4580–4588.
- 10
- 10aW. Li, J. Yang, Z. Wu, J. Wang, B. Li, S. Feng, Y. Deng, F. Zhang, D. Zhao, J. Am. Chem. Soc. 2012, 134, 11864–11867;
- 10bJ. Livage, M. Henry, C. Sanchez, Prog. Solid State Chem. 1988, 18, 259–341;
- 10cH. Liu, W. Li, D. Shen, D. Zhao, G. Wang, J. Am. Chem. Soc. 2015, 137, 13161–13166.
- 11E. Kang, Y. S. Jung, G.-H. Kim, J. Chun, U. Wiesner, A. C. Dillon, J. K. Kim, J. Lee, Adv. Funct. Mater. 2011, 21, 4349–4357.
- 12
- 12aP. D. Yang, D. Y. Zhao, D. I. Margolese, B. F. Chmelka, G. D. Stucky, Nature 1998, 396, 152–155;
- 12bF. Schüth, Chem. Mater. 2001, 13, 3184–3195.
- 13
- 13aJ. Lee, M. C. Orilall, S. C. Warren, M. Kamperman, F. J. DiSalvo, U. Wiesner, Nat. Mater. 2008, 7, 222–228;
- 13bY. Zhu, Y. Zhao, J. Ma, X. Cheng, J. Xie, P. Xu, H. Liu, H. Liu, H. Zhang, M. Wu, A. A. Elzatahry, A. Alghamdi, Y. Deng, D. Zhao, J. Am. Chem. Soc. 2017, 139, 10365–10373;
- 13cC. Li, Ö. Dag, T. D. Dao, T. Nagao, Y. Sakamoto, T. Kimura, O. Terasaki, Y. Yamauchi, Nat. Commun. 2015, 6, 6608.
- 14
- 14aX. Li, M. Qu, Y. Huai, Z. Yu, Electrochim. Acta 2010, 55, 2978–2982;
- 14bS.-W. Han, J. H. Ryu, J. Jeong, D.-H. Yoon, J. Alloys Compd. 2013, 570, 144–149.
- 15A. D. Krawitz, Introduction to Diffraction in Materials Science and Engineering (Ed.: ), Wiley-VCH, Weinheim, 2001, pp. 424, ISBN 0-471-24724-24723.
- 16T. Yu, Y. H. Deng, L. Wang, R. L. Liu, L. J. Zhang, B. Tu, D. Y. Zhao, Adv. Mater. 2007, 19, 2301–2306.
- 17
- 17aH. Song, T. G. Jeong, Y. H. Moon, H. H. Chun, K. Y. Chung, H. S. Kim, B. W. Cho, Y. T. Kim, Sci. Rep. 2014, 4, 4350;
- 17bW. Zhou, W. Li, J.-Q. Wang, Y. Qu, Y. Yang, Y. Xie, K. Zhang, L. Wang, H. Fu, D. Zhao, J. Am. Chem. Soc. 2014, 136, 9280–9283;
- 17cZ. Li, J. Zhang, B. Guan, D. Wang, L. M. Liu, X. W. Lou, Nat. Commun. 2016, 7, 13065.
- 18
- 18aM. G. Verde, L. Baggetto, N. Balke, G. M. Veith, J. K. Seo, Z. Wang, Y. S. Meng, ACS Nano 2016, 10, 4312–4321;
- 18bC. Kim, N. S. Norberg, C. T. Alexander, R. Kostecki, J. Cabana, Adv. Funct. Mater. 2013, 23, 1214–1222;
- 18cM. Wagemaker, D. R. Simon, E. M. Kelder, J. Schoonman, C. Ringpfeil, U. Haake, D. Lützenkirchen-Hecht, R. Frahm, F. M. Mulder, Adv. Mater. 2006, 18, 3169–3173.