Guanitrypmycin Biosynthetic Pathways Imply Cytochrome P450 Mediated Regio- and Stereospecific Guaninyl-Transfer Reactions
Graphical Abstract
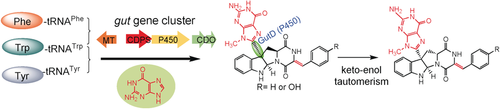
Novel pyrroloindoline alkaloids: Eight rare natural products were identified and their biosynthetic pathways were elucidated by heterologous expression of two gene clusters. Cytochrome P450s as key enzymes catalyze the regio- and stereospecific transfer of a guaninyl moiety to C3 of the indole ring in cyclo-l-Trp-l-Phe (Tyr). Non-enzymatic epimerization via keto–enol tautomerism further increases structural diversity.
Abstract
Mining microbial genomes including those of Streptomyces reveals the presence of a large number of biosynthetic gene clusters. Unraveling this genetic potential has proved to be a useful approach for novel compound discovery. Here, we report the heterologous expression of two similar P450-associated cyclodipeptide synthase-containing gene clusters in Streptomyces coelicolor and identification of eight rare and novel natural products, the C3-guaninyl indole alkaloids guanitrypmycins. Expression of different gene combinations proved that the cyclodipeptide synthases assemble cyclo-l-Trp-l-Phe and cyclo-l-Trp-l-Tyr, which are consecutively and regiospecifically modified by cyclodipeptide oxidases, cytochrome P450 enzymes, and N-methyltransferases. In vivo and in vitro results proved that the P450 enzymes function as key biocatalysts and catalyze the regio- and stereospecific 3α-guaninylation at the indole ring of the tryptophanyl moiety. Isotope-exchange experiments provided evidence for the non-enzymatic epimerization of the biosynthetic pathway products via keto–enol tautomerism. This post-pathway modification during cultivation further increases the structural diversity of guanitrypmycins.
Conflict of interest
The authors declare no conflict of interest.