Near-Infrared-Light-Triggered Antimicrobial Indium Phosphide Quantum Dots
Graphical Abstract
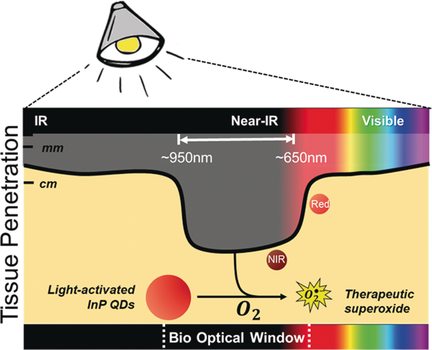
Near-infrared-active quantum dots (QDs) have been designed to generate therapeutic superoxide radicals within pathogens. The combination of activation using a deep-tissue-penetrating light source, and selective mechanism of action, produces an effective treatment. These heavy-metal-free InP QDs successfully eliminate a multidrug-resistant critical pathogen, while leaving human cells unharmed in vitro.
Abstract
The emergence of multidrug-resistant (MDR) pathogens represents one of the most urgent global public health crises. Light-activated quantum dots (QDs) are alternative antimicrobials, with efficient transport, low cost, and therapeutic efficacy, and they can act as antibiotic potentiators, with a mechanism of action orthogonal to small-molecule drugs. Furthermore, light-activation enhances control over the spatiotemporal release and dose of the therapeutic superoxide radicals from QDs. However, the limited deep-tissue penetration of visible light needed for QD activation, and concern over trace heavy metals, have prevented further translation. Herein, we report two indium phosphide (InP) QDs that operate in the near-infrared and deep-red light window, enabling deeper tissue penetration. These heavy-metal-free QDs eliminate MDR pathogenic bacteria, while remaining non-toxic to host human cells. This work provides a pathway for advancing QD nanotherapeutics to combat MDR superbugs.