Promoting Subordinate, Efficient Ruthenium Sites with Interstitial Silicon for Pt-Like Electrocatalytic Activity
Graphical Abstract
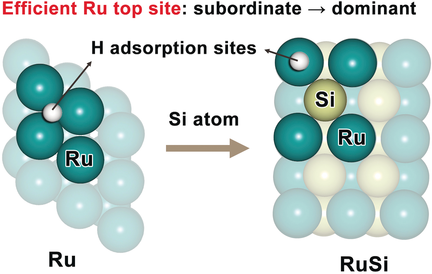
Due for a promotion: Catalytic site preference can be manipulated for the rational design of advanced catalysts. In Ru-based electrocatalysts for the hydrogen evolution reaction, RuSi is identified as a Pt-like electrocatalyst, due to the promotion of the Ru top sites, which are efficient but subordinate in the Ru(0001) catalyst surface.
Abstract
The fundamental understanding and rational manipulation of catalytic site preference at extended solid surfaces is crucial in the search for advanced catalysts. Herein we find that the Ru top sites at metallic ruthenium surface have efficient Pt-like activity for the hydrogen evolution reaction (HER), but they are subordinate to their adjacent, less active Ru3-hollow sites due to the stronger hydrogen-binding ability of the latter. We also present an interstitial incorporation strategy for the promotion of the Ru top sites from subordinate to dominant character, while maintaining Pt-like catalytic activity. Our combined theoretical and experimental studies further identify intermetallic RuSi as a highly active, non-Pt material for catalyzing the HER, because of its suitable electronic structure governed by a good balance of ligand and strain effects.