The Proteome-Wide Potential for Reversible Covalency at Cysteine
Kristine Senkane
Department of Chemistry, The Scripps Research Institute, La Jolla, CA, 92037 USA
Search for more papers by this authorCorresponding Author
Dr. Ekaterina V. Vinogradova
Department of Chemistry, The Scripps Research Institute, La Jolla, CA, 92037 USA
Search for more papers by this authorDr. Radu M. Suciu
Department of Chemistry, The Scripps Research Institute, La Jolla, CA, 92037 USA
Search for more papers by this authorDr. Vincent M. Crowley
Department of Chemistry, The Scripps Research Institute, La Jolla, CA, 92037 USA
Search for more papers by this authorDr. Balyn W. Zaro
Department of Chemistry, The Scripps Research Institute, La Jolla, CA, 92037 USA
Search for more papers by this authorDr. J. Michael Bradshaw
Principia Biopharma, 220 E. Grand Avenue, South San Francisco, CA, 94080 USA
Search for more papers by this authorDr. Ken A. Brameld
Principia Biopharma, 220 E. Grand Avenue, South San Francisco, CA, 94080 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Benjamin F. Cravatt
Department of Chemistry, The Scripps Research Institute, La Jolla, CA, 92037 USA
Search for more papers by this authorKristine Senkane
Department of Chemistry, The Scripps Research Institute, La Jolla, CA, 92037 USA
Search for more papers by this authorCorresponding Author
Dr. Ekaterina V. Vinogradova
Department of Chemistry, The Scripps Research Institute, La Jolla, CA, 92037 USA
Search for more papers by this authorDr. Radu M. Suciu
Department of Chemistry, The Scripps Research Institute, La Jolla, CA, 92037 USA
Search for more papers by this authorDr. Vincent M. Crowley
Department of Chemistry, The Scripps Research Institute, La Jolla, CA, 92037 USA
Search for more papers by this authorDr. Balyn W. Zaro
Department of Chemistry, The Scripps Research Institute, La Jolla, CA, 92037 USA
Search for more papers by this authorDr. J. Michael Bradshaw
Principia Biopharma, 220 E. Grand Avenue, South San Francisco, CA, 94080 USA
Search for more papers by this authorDr. Ken A. Brameld
Principia Biopharma, 220 E. Grand Avenue, South San Francisco, CA, 94080 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Benjamin F. Cravatt
Department of Chemistry, The Scripps Research Institute, La Jolla, CA, 92037 USA
Search for more papers by this authorGraphical Abstract
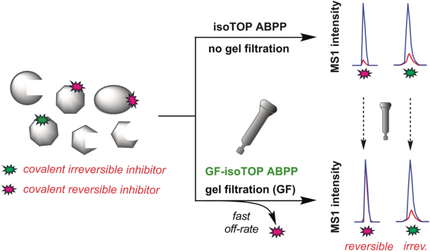
A chemical proteomic platform that integrates gel filtration with activity-based protein profiling (ABPP) provides a method to assess small-molecule electrophiles for reversible versus irreversible interactions with cysteine residues in native biological systems, revealing the broad potential for reversible covalency as a strategy for chemical-probe discovery.
Abstract
Reversible covalency, achieved with, for instance, highly electron-deficient olefins, offers a compelling strategy to design chemical probes and drugs that benefit from the sustained target engagement afforded by irreversible compounds, while avoiding permanent protein modification. Reversible covalency has mainly been evaluated for cysteine residues in individual kinases and the broader potential for this strategy to engage cysteines across the proteome remains unexplored. Herein, we describe a mass-spectrometry-based platform that integrates gel filtration with activity-based protein profiling to assess cysteine residues across the human proteome for both irreversible and reversible interactions with small-molecule electrophiles. Using this method, we identify numerous cysteine residues from diverse protein classes that are reversibly engaged by cyanoacrylamide fragment electrophiles, revealing the broad potential for reversible covalency as a strategy for chemical-probe discovery.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201905829-sup-0001-misc_information.pdf7.1 MB | Supplementary |
| anie201905829-sup-0001-Table 1.xlsx1.2 MB | Supplementary |
| anie201905829-sup-0001-Table 2.xlsx61.9 KB | Supplementary |
| anie201905829-sup-0001-Table 3.xlsx33.8 KB | Supplementary |
| anie201905829-sup-0001-Table 4.xlsx39.4 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. Singh, R. C. Petter, T. A. Baillie, A. Whitty, Nat. Rev. Drug Discovery 2011, 10, 307–317.
- 2Q. S. Liu, Y. Sabnis, Z. Zhao, T. H. Zhang, S. J. Buhrlage, L. H. Jones, N. S. Gray, Chem. Biol. 2013, 20, 146–159.
- 3R. A. Bauer, Drug Discovery Today 2015, 20, 1061–1073.
- 4D. S. Johnson, E. Weerapana, B. F. Cravatt, Future Med. Chem. 2010, 2, 949–964.
- 5J. C. Byrd, B. Harrington, S. O'Brien, J. A. Jones, A. Schuh, S. Devereux, J. Chaves, W. G. Wierda, F. T. Awan, J. R. Brown, P. Hillmen, D. M. Stephens, P. Ghia, J. C. Barrientos, J. M. Pagel, J. Woyach, D. Johnson, J. Huang, X. Wang, A. Kaptein, B. J. Lannutti, T. Covey, M. Fardis, J. McGreivy, A. Hamdy, W. Rothbaum, R. Izumi, T. G. Diacovo, A. J. Johnson, R. R. Furman, N. Engl. J. Med. 2016, 374, 323–332.
- 6L. A. Honigberg, A. M. Smith, M. Sirisawad, E. Verner, D. Loury, B. Chang, S. Li, Z. Pan, D. H. Thamm, R. A. Miller, J. J. Buggy, Proc. Natl. Acad. Sci. USA 2010, 107, 13075–13080.
- 7P. Maione, A. Rossi, M. Bareschino, P. C. Sacco, C. Schettino, F. Casaluce, A. Sgambato, C. Gridelli, Curr. Pharm. Des. 2014, 20, 3894–3900.
- 8R. Mah, J. R. Thomas, C. M. Shafer, Bioorg. Med. Chem. Lett. 2014, 24, 33–39.
- 9S. Nakayama, R. Atsumi, H. Takakusa, Y. Kobayashi, A. Kurihara, Y. Nagai, D. Nakai, O. Okazaki, Drug Metab. Dispos. 2009, 37, 1970–1977.
- 10S. M. Hacker, K. M. Backus, M. R. Lazear, S. Forli, B. E. Correia, B. F. Cravatt, Nat. Chem. 2017, 9, 1181–1190.
- 11K. M. Backus, B. E. Correia, K. M. Lum, S. Forli, B. D. Horning, G. E. Gonzalez-Paez, S. Chatterjee, B. R. Lanning, J. R. Teijaro, A. J. Olson, D. W. Wolan, B. F. Cravatt, Nature 2016, 534, 570–574.
- 12Y. C. Chen, C. Zhang, Genes Cancer 2016, 7, 148–153.
- 13S. Niessen, M. M. Dix, S. Barbas, Z. E. Potter, S. Lu, O. Brodsky, S. Planken, D. Behenna, C. Almaden, K. S. Gajiwala, K. Ryan, R. Ferre, M. R. Lazear, M. M. Hayward, J. C. Kath, B. F. Cravatt, Cell Chem. Biol. 2017, 24, 1388–1400.
- 14J. W. Chang, A. B. Cognetta 3rd, M. J. Niphakis, B. F. Cravatt, ACS Chem. Biol. 2013, 8, 1590–1599.
- 15T. Barf, T. Covey, R. Izumi, B. van de Kar, M. Gulrajani, B. van Lith, M. van Hoek, E. de Zwart, D. Mittag, D. Demont, S. Verkaik, F. Krantz, P. G. Pearson, R. Ulrich, A. Kaptein, J. Pharmacol. Exp. Ther. 2017, 363, 240–252.
- 16N. Shindo, H. Fuchida, M. Sato, K. Watari, T. Shibata, K. Kuwata, C. Miura, K. Okamoto, Y. Hatsuyama, K. Tokunaga, S. Sakamoto, S. Morimoto, Y. Abe, M. Shiroishi, J. M. M. Caaveiro, T. Ueda, T. Tamura, N. Matsunaga, T. Nakao, S. Koyanagi, S. Ohdo, Y. Yamaguchi, I. Hamachi, M. Ono, A. Ojida, Nat. Chem. Biol. 2019, 15, 250–258.
- 17B. W. Zaro, L. R. Whitby, K. M. Lum, B. F. Cravatt, J. Am. Chem. Soc. 2016, 138, 15841–15844.
- 18I. M. Serafimova, M. A. Pufall, S. Krishnan, K. Duda, M. S. Cohen, R. L. Maglathlin, J. M. McFarland, R. M. Miller, M. Frodin, J. Taunton, Nat. Chem. Biol. 2012, 8, 471–476.
- 19R. M. Miller, V. O. Paavilainen, S. Krishnan, I. M. Serafimova, J. Taunton, J. Am. Chem. Soc. 2013, 135, 5298–5301.
- 20S. Krishnan, R. M. Miller, B. X. Tian, R. D. Mullins, M. P. Jacobson, J. Taunton, J. Am. Chem. Soc. 2014, 136, 12624–12630.
- 21N. London, R. M. Miller, S. Krishnan, K. Uchida, J. J. Irwin, O. Eidam, L. Gibold, P. Cimermancic, R. Bonnet, B. K. Shoichet, J. Taunton, Nat. Chem. Biol. 2014, 10, 1066–1072.
- 22J. M. Bradshaw, J. M. McFarland, V. O. Paavilainen, A. Bisconte, D. Tam, V. T. Phan, S. Romanov, D. Finkle, J. Shu, V. Patel, T. Ton, X. Y. Li, D. G. Loughhead, P. A. Nunn, D. E. Karr, M. E. Gerritsen, J. O. Funk, T. D. Owens, E. Verner, K. A. Brameld, R. J. Hill, D. M. Goldstein, J. Taunton, Nat. Chem. Biol. 2015, 11, 525–531.
- 23A. Bandyopadhyay, J. M. Gao, Curr. Opin. Chem. Biol. 2016, 34, 110–116.
- 24M. H. Potashman, M. E. Duggan, J. Med. Chem. 2009, 52, 1231–1246.
- 25T. A. Baillie, Angew. Chem. Int. Ed. 2016, 55, 13408–13421; Angew. Chem. 2016, 128, 13606–13619.
- 26R. B. Perni, S. J. Almquist, R. A. Byrn, G. Chandorkar, P. R. Chaturvedi, L. F. Courtney, C. J. Decker, K. Dinehart, C. A. Gates, S. L. Harbeson, A. Heiser, G. Kalkeri, E. Kolaczkowski, K. Lin, Y. P. Luong, B. G. Rao, W. P. Taylor, J. A. Thomson, R. D. Tung, Y. Y. Wei, A. D. Kwong, C. Lin, Antimicrob. Agents Chemother. 2006, 50, 899–909.
- 27S. Venkatraman, S. L. Bogen, A. Arasappan, F. Bennett, K. Chen, E. Jao, Y. T. Liu, R. Lovey, S. Hendrata, Y. H. Huang, W. D. Pan, T. Parekh, P. Pinto, V. Popov, R. Pike, S. Ruan, B. Santhanam, B. Vibulbhan, W. L. Wu, W. Y. Yang, J. S. Kong, X. Liang, J. Wong, R. Liu, N. Butkiewicz, R. Chase, A. Hart, S. Agrawal, P. Ingravallo, J. Pichardo, R. Kong, B. Baroudy, B. Malcolm, Z. Y. Guo, A. Prongay, V. Madison, L. Broske, X. M. Cui, K. C. Cheng, Y. S. Hsieh, J. M. Brisson, D. Prelusky, W. Korfmacher, R. White, S. Bogdanowich-Knipp, A. Pavlovsky, P. Bradley, A. K. Saksena, A. Ganguly, J. Piwinski, V. Girijavallabhan, F. G. Njoroge, J. Med. Chem. 2006, 49, 6074–6086.
- 28D. L. Boger, H. Sato, A. E. Lerner, M. P. Hedrick, R. A. Fecik, H. Miyauchi, G. D. Wilkie, B. J. Austin, M. P. Patricelli, B. F. Cravatt, Proc. Natl. Acad. Sci. USA 2000, 97, 5044–5049.
- 29J. Adams, M. Kauffman, Cancer Invest. 2004, 22, 304–311.
- 30A. Berteotti, F. Vacondio, A. Lodola, M. Bassi, C. Silva, M. Mor, A. Cavalli, ACS Med. Chem. Lett. 2014, 5, 501–505.
- 31C. Mathieu, E. Degrande, Vasc. Health Risk Manage. 2008, 4, 1349–1360.
- 32D. J. Augeri, J. A. Robl, D. A. Betebenner, D. R. Magnin, A. Khanna, J. G. Robertson, A. Wang, L. M. Simpkins, P. Taunk, Q. Huang, S.-P. Han, B. Abboa-Offei, M. Cap, L. Xin, L. Tao, E. Tozzo, G. E. Welzel, D. M. Egan, J. Marcinkeviciene, S. Y. Chang, S. A. Biller, M. S. Kirby, R. A. Parker, L. G. Hamann, J. Med. Chem. 2005, 48, 5025–5037.
- 33R. J. Pearson, D. G. Blake, M. Mezna, P. M. Fischer, N. J. Westwood, C. McInnes, Cell Chem. Biol. 2018, 25, 1107–1116.
- 34G. Akçay, M. A. Belmonte, B. Aquila, C. Chuaqui, A. W. Hird, M. L. Lamb, P. B. Rawlins, N. Su, S. Tentarelli, N. P. Grimster, Q. Su, Nat. Chem. Biol. 2016, 12, 931–936.
- 35L. Bar-Peled, E. K. Kemper, R. M. Suciu, E. V. Vinogradova, K. M. Backus, B. D. Horning, T. A. Paul, T. A. Ichu, R. U. Svensson, J. Olucha, M. W. Chang, B. P. Kok, Z. Zhu, N. T. Ihle, M. M. Dix, P. Jiang, M. M. Hayward, E. Saez, R. J. Shaw, B. F. Cravatt, Cell 2017, 171, 696–709.
- 36X. Zhang, V. M. Crowley, T. G. Wucherpfennig, M. M. Dix, B. F. Cravatt, Nat. Chem. Biol 2019, 15, 737–746.
- 37E. Weerapana, C. Wang, G. M. Simon, F. Richter, S. Khare, M. B. Dillon, D. A. Bachovchin, K. Mowen, D. Baker, B. F. Cravatt, Nature 2010, 468, 790–795.