Introducing Perylene as a New Member to the Azaborine Family
Tanja Kaehler
Institut für Anorganische Chemie, Goethe-Universität Frankfurt, Max-von-Laue-Strasse 7, 60438 Frankfurt (Main), Germany
Search for more papers by this authorDr. Michael Bolte
Institut für Anorganische Chemie, Goethe-Universität Frankfurt, Max-von-Laue-Strasse 7, 60438 Frankfurt (Main), Germany
Search for more papers by this authorDr. Hans-Wolfram Lerner
Institut für Anorganische Chemie, Goethe-Universität Frankfurt, Max-von-Laue-Strasse 7, 60438 Frankfurt (Main), Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Matthias Wagner
Institut für Anorganische Chemie, Goethe-Universität Frankfurt, Max-von-Laue-Strasse 7, 60438 Frankfurt (Main), Germany
Search for more papers by this authorTanja Kaehler
Institut für Anorganische Chemie, Goethe-Universität Frankfurt, Max-von-Laue-Strasse 7, 60438 Frankfurt (Main), Germany
Search for more papers by this authorDr. Michael Bolte
Institut für Anorganische Chemie, Goethe-Universität Frankfurt, Max-von-Laue-Strasse 7, 60438 Frankfurt (Main), Germany
Search for more papers by this authorDr. Hans-Wolfram Lerner
Institut für Anorganische Chemie, Goethe-Universität Frankfurt, Max-von-Laue-Strasse 7, 60438 Frankfurt (Main), Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Matthias Wagner
Institut für Anorganische Chemie, Goethe-Universität Frankfurt, Max-von-Laue-Strasse 7, 60438 Frankfurt (Main), Germany
Search for more papers by this authorGraphical Abstract
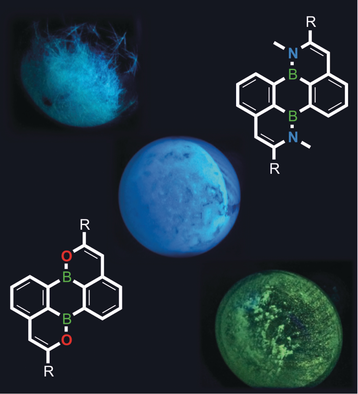
BN/CC isosterism: Doubly BO- and BN-doped perylenes have been prepared, which further expand the chemical space of a ubiquitous building block for organic electronic materials. The key cyclization step proceeds via a quantitative Wacker-type addition reaction of BO−H or BN−H bonds to adjacent alkynyl moieties.
Abstract
Substitutional doping of perylene with two boron atoms at the 6b/12b positions and two oxygen or nitrogen atoms at the 1/7 positions has been achieved. The modular synthesis route developed for these bis-BO- (3) and bis-BN-perylenes (5) starts from the readily accessible borinic acid derivative of the doubly brominated 9,10-dihydro-9,10-diboraanthracene (DBA), 1,5-Br2DBA(OH)2. A Stille-type reaction first furnishes the alkynyl-substituted species 1,5-(RCC)2DBA(OH)2 (2), which undergo double ring closure to afford 3 via the gold-catalyzed addition of the O−H bonds to the C≡C bonds. Treatment of 2 with MeN(SiMe3)2 leads to aminoborane intermediates 1,5-(RCC)2DBA(N(H)Me)2, which can be ring-closed to give 5 in a similar manner as in the case of 3. Different substituents R (such as Me, tBuPh) can be introduced at the 2/8 positions of the perylene core. The products obtained undergo reversible reduction and are efficient blue/turquoise emitters.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201905823-sup-0001-misc_information.pdf14.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. J. D. Bosdet, W. E. Piers, Can. J. Chem. 2009, 87, 8–29;
- 1bX.-Y. Wang, J.-Y. Wang, J. Pei, Chem. Eur. J. 2015, 21, 3528–3539;
- 1cG. Bélanger-Chabot, H. Braunschweig, D. K. Roy, Eur. J. Inorg. Chem. 2017, 4353–4368;
- 1dZ. X. Giustra, S.-Y. Liu, J. Am. Chem. Soc. 2018, 140, 1184–1194.
- 2A. J. V. Marwitz, M. H. Matus, L. N. Zakharov, D. A. Dixon, S.-Y. Liu, Angew. Chem. Int. Ed. 2009, 48, 973–977; Angew. Chem. 2009, 121, 991–995.
- 3E. R. Abbey, L. N. Zakharov, S.-Y. Liu, J. Am. Chem. Soc. 2008, 130, 7250–7252.
- 4R. Islas, E. Chamorro, J. Robles, T. Heine, J. C. Santos, G. Merino, Struct. Chem. 2007, 18, 833–839.
- 5J. S. A. Ishibashi, J. L. Marshall, A. Mazière, G. J. Lovinger, B. Li, L. N. Zakharov, A. Dargelos, A. Graciaa, A. Chrostowska, S.-Y. Liu, J. Am. Chem. Soc. 2014, 136, 15414–15421.
- 6M. J. D. Bosdet, C. A. Jaska, W. E. Piers, T. S. Sorensen, M. Parvez, Org. Lett. 2007, 9, 1395–1398.
- 7
- 7aC. R. McConnel, F. Haeffner, A. W. Baggett, S.-Y. Liu, J. Am. Chem. Soc. 2019, 141, 9072–9078;
- 7bC. R. McConnel, S.-Y. Liu, Chem. Soc. Rev. 2019, https://doi.org/10.1039/c9cs00218a.
- 8G. A. Molander, S. R. Wisniewski, J. Org. Chem. 2014, 79, 6663–6678.
- 9F. Sun, L. Lv, M. Huang, Z. Zhou, X. Fang, Org. Lett. 2014, 16, 5024–5027.
- 10
- 10aA. Vlasceanu, M. Jessing, J. P. Kilburn, Bioorg. Med. Chem. 2015, 23, 4453–4461;
- 10bP. Zhao, D. O. Nettleton, R. G. Karki, F. J. Zécri, S.-Y. Liu, ChemMedChem 2017, 12, 358–361.
- 11
- 11aJ. A. Bailey, M. Ploeger, P. G. Pringle, Inorg. Chem. 2014, 53, 7763–7769;
- 11bS. Xu, F. Haeffner, B. Li, L. N. Zakharov, S.-Y. Liu, Angew. Chem. Int. Ed. 2014, 53, 6795–6799; Angew. Chem. 2014, 126, 6913–6917;
- 11cF. Sun, M. Huang, Z. Zhou, X. Fang, RSC Adv. 2015, 5, 75607–75611;
- 11dS. Xu, Y. Zhang, B. Li, S.-Y. Liu, J. Am. Chem. Soc. 2016, 138, 14566–14569;
- 11eC. R. McConnell, P. G. Campbell, C. R. Fristoe, P. Memmel, L. N. Zakharov, B. Li, C. Darrigan, A. Chrostowska, S.-Y. Liu, Eur. J. Inorg. Chem. 2017, 2207–2210.
- 12
- 12aT. Hatakeyama, S. Hashimoto, S. Seki, M. Nakamura, J. Am. Chem. Soc. 2011, 133, 18614–18617;
- 12bX. Wang, F. Zhang, J. Liu, R. Tang, Y. Fu, D. Wu, Q. Xu, X. Zhuang, G. He, X. Feng, Org. Lett. 2013, 15, 5714–5717;
- 12cX.-Y. Wang, F.-D. Zhuang, R.-B. Wang, X.-C. Wang, X.-Y. Cao, J.-Y. Wang, J. Pei, J. Am. Chem. Soc. 2014, 136, 3764–3767;
- 12dA. W. Baggett, F. Guo, B. Li, S.-Y. Liu, F. Jäkle, Angew. Chem. Int. Ed. 2015, 54, 11191–11195; Angew. Chem. 2015, 127, 11343–11347;
- 12eS. Wang, D.-T. Yang, J. Lu, H. Shimogawa, S. Gong, X. Wang, S. K. Mellerup, A. Wakamiya, Y.-L. Chang, C. Yang, Z.-H. Lu, Angew. Chem. Int. Ed. 2015, 54, 15074–15078; Angew. Chem. 2015, 127, 15289–15293.
- 13Borylative cyclization:
- 13aM. J. S. Dewar, V. P. Kubba, R. Pettit, J. Chem. Soc. 1958, 3073–3076;
- 13bT. Hatakeyama, S. Hashimoto, T. Oba, M. Nakamura, J. Am. Chem. Soc. 2012, 134, 19600–19603;
- 13cJ.-S. Lu, S.-B. Ko, N. R. Walters, Y. Kang, F. Sauriol, S. Wang, Angew. Chem. Int. Ed. 2013, 52, 4544–4548; Angew. Chem. 2013, 125, 4642–4646;
- 13dA. Issaian, K. N. Tu, S. A. Blum, Acc. Chem. Res. 2017, 50, 2598–2609;
- 13eF.-D. Zhuang, J.-M. Han, S. Tang, J.-H. Yang, Q.-R. Chen, J.-Y. Wang, J. Pei, Organometallics 2017, 36, 2479–2482;
- 13fG. H. M. Davies, A. Mukhtar, B. Saeednia, F. Sherafat, C. B. Kelly, G. A. Molander, J. Org. Chem. 2017, 82, 5380–5390;
- 13gJ. S. A. Ishibashi, C. Darrigan, A. Chrostowska, B. Li, S.-Y. Liu, Dalton Trans. 2019, 48, 2807–2812; boron insertion:
- 13hS. Tsuchiya, H. Saito, K. Nogi, H. Yorimitsu, Org. Lett. 2019, 21, 3855–3860; ring-closing metathesis:
- 13iA. D. Rohr, J. W. Kampf, A. J. Ashe III, Organometallics 2014, 33, 1318–1321;
- 13jA. N. Brown, B. Li, S.-Y. Liu, J. Am. Chem. Soc. 2015, 137, 8932–8935;
- 13kA. Abengózar, P. García-García, D. Sucunza, L. M. Frutos, O. Castaño, D. Sampedro, A. Pérez-Redondo, J. J. Vaquero, Org. Lett. 2017, 19, 3458–3461;
- 13lZ. Liu, J. S. A. Ishibashi, C. Darrigan, A. Dargelos, A. Chrostowska, B. Li, M. Vasiliu, D. A. Dixon, S.-Y. Liu, J. Am. Chem. Soc. 2017, 139, 6082–6085.
- 14M. J. D. Bosdet, W. E. Piers, T. S. Sorensen, M. Parvez, Angew. Chem. Int. Ed. 2007, 46, 4940–4943; Angew. Chem. 2007, 119, 5028–5031.
- 15F. Alonso, I. P. Beletskaya, M. Yus, Chem. Rev. 2004, 104, 3079–3159.
- 16Singly and doubly benzannulated bis-BN-perylenes have been reported by Piers et al. and Bettinger et al., respectively:
- 16aM. J. D. Bosdet, W. E. Piers, T. S. Sorensen, M. Parvez, Can. J. Chem. 2010, 88, 426–433;
- 16bM. Müller, S. Behnle, C. Maichle-Mössmer, H. F. Bettinger, Chem. Commun. 2014, 50, 7821–7823.
- 17F. Würthner, Chem. Commun. 2004, 1564–1579.
- 18
- 18aX. Zhan, A. Facchetti, S. Barlow, T. J. Marks, M. A. Ratner, M. R. Wasielewski, S. R. Marder, Adv. Mater. 2011, 23, 268–284;
- 18bF. Würthner, C. R. Saha-Möller, B. Fimmel, S. Ogi, P. Leowanawat, D. Schmidt, Chem. Rev. 2016, 116, 962–1052.
- 19J. M. Farrell, D. Schmidt, V. Grande, F. Würthner, Angew. Chem. Int. Ed. 2017, 56, 11846–11850; Angew. Chem. 2017, 129, 12008–12012.
- 20C.-W. Wang, L. Yang, C. Zhu, J.-G. Yu, S.-H. Lin, J. Chem. Phys. 2014, 141, 084106.
- 21S. Brend'amour, J. Gilmer, M. Bolte, H.-W. Lerner, M. Wagner, Chem. Eur. J. 2018, 24, 16910–16918.
- 22S. Luliński, J. Smętek, K. Durka, J. Serwatowski, Eur. J. Org. Chem. 2013, 8315–8322.
- 23In the context of arylboranes, Pd-catalyzed C−C-coupling protocols have previously been applied almost exclusively to sterically protected (for example, mesitylated) species, which preclude further derivatization at the B site:
- 23aS. Yamaguchi, T. Shirasaka, K. Tamao, Org. Lett. 2000, 2, 4129–4132;
- 23bX. Y. Liu, D. R. Bai, S. Wang, Angew. Chem. Int. Ed. 2006, 45, 5475–5478; Angew. Chem. 2006, 118, 5601–5604;
- 23cC. Reus, S. Weidlich, M. Bolte, H.-W. Lerner, M. Wagner, J. Am. Chem. Soc. 2013, 135, 12892–12907;
- 23dF. Guo, X. Yin, F. Pammer, F. Cheng, D. Fernandez, R. A. Lalancette, F. Jäkle, Macromolecules 2014, 47, 7831–7841;
- 23eA. John, M. Bolte, H. W. Lerner, M. Wagner, Angew. Chem. Int. Ed. 2017, 56, 5588–5592; Angew. Chem. 2017, 129, 5680–5684; a rare example of successful Stille-type reactions carried out on iodinated (still sterically encumbered) diarylborinic acids has been provided: W.-M. Wan, F. Cheng, F. Jäkle, Angew. Chem. Int. Ed. 2014, 53, 8934–8938; Angew. Chem. 2014, 126, 9080–9084.
- 24C. Körner, P. Starkov, T. D. Sheppard, J. Am. Chem. Soc. 2010, 132, 5968–5969.
- 25DBA(OH)2: B−O 1.387(4) and 1.377(4) Å, 1,5-Br2DBA(OH)2: B−O 1.357(5) Å; DBA(NH2)2: B−N 1.389(4) Å.
- 25aE. Januszewski, A. Lorbach, R. Grewal, M. Bolte, J. W. Bats, H.-W. Lerner, M. Wagner, Chem. Eur. J. 2011, 17, 12696–12705;
- 25bK. Durka, S. Luliński, K. N. Jarzembska, J. Smętek, J. Serwatowski, K. Woźniak, Acta Crystallogr. Sect. B 2014, 70, 157–171;
- 25cP. Müller, S. Huck, H. Köppel, H. Pritzkow, W. Siebert, Z. Naturforsch. B 1995, 50, 1476–1484.
- 26B−N bond elongation is also observed for 5 a (av. 1.433 Å) and 5 c (av. 1.439 Å).
- 27A. John, S. Kirschner, M. K. Fengel, M. Bolte, H.-W. Lerner, M. Wagner, Dalton Trans. 2019, 48, 1871–1877.
- 28I. M. Hunsberger, R. Ketcham, H. S. Gutowsky, J. Am. Chem. Soc. 1952, 74, 4839–4845.
- 29G. U. Kulkarni, A. Ranganathan, Proc. Indian Acad. Sci. (Chem. Sci.) 2003, 115, 637–647.
10.1007/BF02708224 Google Scholar
- 30A second, albeit irreversible, redox event is observable for 3 a, 3 d, and 5 a at peak potentials of Epc=−3.03 V, −2.88 V, and −3.25 V. In the cases of 5 c and 5 d, already the first reduction is irreversible (Epc=−2.70 V and −2.70 V; see the Supporting Information for more details).
- 31In the cases of vibrationally fine-structured absorption/emission bands, λmax/λem refer to the most bathochromic/hypsochromic local maximum.
- 32“Principles of Fluorescence Spectroscopy”: J. R. Lakowicz, 3rd edn, Springer, New York, 2006.
- 33EGopt=1240/λonset; each λonset was determined by constructing a tangent at the point of inflection of the bathochromic slope of the most red-shifted absorption maximum.
- 34D. Casanova, Int. J. Quantum Chem. 2015, 115, 442–452.