Neutral Organic Super Electron Donors Made Catalytic
Correction(s) for this article
-
Corrigendum: Neutral Organic Super Electron Donors Made Catalytic
- Volume 58Issue 43Angewandte Chemie International Edition
- pages: 15183-15183
- First Published online: October 14, 2019
Simon Rohrbach
Dept. of Pure and Applied Chemistry, University of Strathclyde, 295 Cathedral Street, Glasgow, G1 1XL UK
Search for more papers by this authorDr. Rushabh S. Shah
GlaxoSmithKline Medicines Research Centre, Gunnels Wood Road, Stevenage, SG1 2NY UK
Search for more papers by this authorCorresponding Author
Prof. Dr. Tell Tuttle
Dept. of Pure and Applied Chemistry, University of Strathclyde, 295 Cathedral Street, Glasgow, G1 1XL UK
Search for more papers by this authorCorresponding Author
Prof. Dr. John A. Murphy
Dept. of Pure and Applied Chemistry, University of Strathclyde, 295 Cathedral Street, Glasgow, G1 1XL UK
Search for more papers by this authorSimon Rohrbach
Dept. of Pure and Applied Chemistry, University of Strathclyde, 295 Cathedral Street, Glasgow, G1 1XL UK
Search for more papers by this authorDr. Rushabh S. Shah
GlaxoSmithKline Medicines Research Centre, Gunnels Wood Road, Stevenage, SG1 2NY UK
Search for more papers by this authorCorresponding Author
Prof. Dr. Tell Tuttle
Dept. of Pure and Applied Chemistry, University of Strathclyde, 295 Cathedral Street, Glasgow, G1 1XL UK
Search for more papers by this authorCorresponding Author
Prof. Dr. John A. Murphy
Dept. of Pure and Applied Chemistry, University of Strathclyde, 295 Cathedral Street, Glasgow, G1 1XL UK
Search for more papers by this authorGraphical Abstract
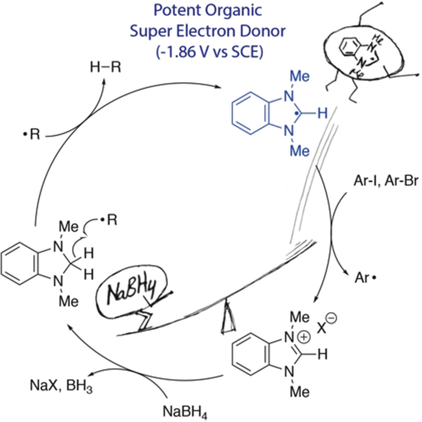
CatalySED! An organocatalytic role for a benzimidazolium salt in radical chemistry is reported. A highly reducing intermediate (−1.86 V vs. SCE) is produced simply by treatment with NaBH4 and then using air as initiator. This is the first time that an organic super electron donor has been used catalytically, which introduces a novel catalytic approach for the upconversion of reducing power.
Abstract
Neutral organic super electron donors (SEDs) display impressive reducing power but, until now, it has not been possible to use them catalytically in radical chain reactions. This is because, following electron transfer, these donors form persistent radical cations that trap substrate-derived radicals. This paper unlocks a conceptually new approach to super electron donors that overcomes this issue, leading to the first catalytic neutral organic super electron donor.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201905814-sup-0001-misc_information.pdf5.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. A. Murphy, C. Lampard, N. Lewis, J. Chem. Soc. Chem. Commun. 1993, 295–297;
- 1b“The Radical-Polar Crossover Reaction”: J. A. Murphy, in Radicals in Organic Synthesis, Vol. 1 (Eds.: ), Wiley-VCH, Weinheim, chapter 2.7, 2001.
- 2
- 2aC. Burkholder, W. R. Dolbier, Jr., M. Médebielle, J. Org. Chem. 1998, 63, 5385–5394;
- 2bM. Médebielle, W. R. Dolbier, Jr., J. Fluorine Chem. 2008, 129, 930–942;
- 2cM. Mohan, J. A. Murphy, F. LeStrat, H. P. Wessel, Beilstein J. Org. Chem. 2009, 5, 1.
- 3
- 3aJ. A. Murphy, T. A. Khan, S.-Z. Zhou, D. W. Thomson, M. Mahesh, Angew. Chem. Int. Ed. 2005, 44, 1356–1360; Angew. Chem. 2005, 117, 1380–1384;
- 3bJ. A. Murphy, S.-Z. Zhou, D. W. Thomson, F. Schoenebeck, M. Mahesh, S. R. Park, T. Tuttle, L. E. A. Berlouis, Angew. Chem. Int. Ed. 2007, 46, 5178–5183; Angew. Chem. 2007, 119, 5270–5275;
- 3cJ. A. Murphy, J. Garnier, S. R. Park, F. Schoenebeck, S.-Z. Zhou, A. T. Turner, Org. Lett. 2008, 10, 1227–1230;
- 3dS. S. Hanson, E. Doni, K. T. Traboulsee, G. Coulthard, J. A. Murphy, C. A. Dyker, Angew. Chem. Int. Ed. 2015, 54, 11236–11239; Angew. Chem. 2015, 127, 11388–11391;
- 3eJ. D. Martin, C. A. Dyker, Can. J. Chem. 2018, 96, 522–525;
- 3fS. S. Hanson, N. A. Richard, C. A. Dyker, Chem. Eur. J. 2015, 21, 8052–8055.
- 4For reviews, see
- 4aE. Doni, J. A. Murphy, Chem. Commun. 2014, 50, 6073–6087;
- 4bJ. A. Murphy, J. Org. Chem. 2014, 79, 3731–3746;
- 4cJ. Broggi, T. Terme, P. Vanelle, Angew. Chem. Int. Ed. 2014, 53, 384–413; Angew. Chem. 2014, 126, 392–423.
- 5
- 5aE. Cahard, F. Schoenebeck, J. Garnier, S. P. Y. Cutulic, S. Zhou, J. A. Murphy, Angew. Chem. Int. Ed. 2012, 51, 3673–3676; Angew. Chem. 2012, 124, 3733–3736;
- 5bE. Doni, B. Mondal, S. O'Sullivan, T. Tuttle, J. A. Murphy, J. Am. Chem. Soc. 2013, 135, 10934–10937;
- 5cS. O'Sullivan, E. Doni, T. Tuttle, J. A. Murphy, Angew. Chem. Int. Ed. 2014, 53, 474–478; Angew. Chem. 2014, 126, 484–488.
- 6Organic electron donors are diverse in their structures and application, for example,
- 6aG. Tintori, P. Nabokoff, R. Buhaibeh, D. Bergé-Lefranc, S. Redon, J. Broggi, P. Vanelle, Angew. Chem. Int. Ed. 2018, 57, 3148–3153; Angew. Chem. 2018, 130, 3202–3207;
- 6bQ. Wang, M. Poznik, M. Li, P. J. Walsh, J. J. Chruma, Adv. Synth. Catal. 2018, 360, 2854–2868;
- 6cD. Mandal, R. Dolai, N. Chrysochos, P. Kalita, R. Kumar, D. Dhara, A. Maiti, R. S. Narayanan, G. Rajaraman, C. Schulzke, V. Chandrasekhar, A. Jana, Org. Lett. 2017, 19, 5605–5608;
- 6dM. Brasholz, Angew. Chem. Int. Ed. 2017, 56, 10280–10281; Angew. Chem. 2017, 129, 10414–10415;
- 6eM. Neumann, S. Füldner, B. König, K. Zeitler, Angew. Chem. Int. Ed. 2011, 50, 951–954; Angew. Chem. 2011, 123, 981–985;
- 6fI. Ghosh, B. König, Angew. Chem. Int. Ed. 2016, 55, 7676–7679; Angew. Chem. 2016, 128, 7806–7810;
- 6gB. Eberle, E. Kaifer, H.-J. Himmel, Angew. Chem. Int. Ed. 2017, 56, 3360–3363; Angew. Chem. 2017, 129, 3408–3412;
- 6hJ. Broggi, M. Rollet, J.-L. Clément, G. Canard, T. Terme, D. Gigmes, P. Vanelle, Angew. Chem. Int. Ed. 2016, 55, 5994–5999; Angew. Chem. 2016, 128, 6098–6103;
- 6iH. Herrmann, M. Reinmuth, S. Wiesner, O. Hübner, E. Kaifer, H. Wadepohl, H.-J. Himmel, Eur. J. Inorg. Chem. 2015, 2345–2361.
- 7
- 7aH. Fischer, J. Am. Chem. Soc. 1986, 108, 3925–3927;
- 7bA. Studer, Chem. Eur. J. 2001, 7, 1159–1164.
10.1002/1521-3765(20010316)7:6<1159::AID-CHEM1159>3.0.CO;2-I CAS PubMed Web of Science® Google Scholar
- 8J. A. Murphy, F. Schoenebeck, N. J. Findlay, D. W. Thomson, S.-Z. Zhou, J. Garnier, J. Am. Chem. Soc. 2009, 131, 6475–6479.
- 9
- 9aH. Chikashita, H. Ide, K. Itoh, J. Org. Chem. 1986, 51, 5400–5405;
- 9bD. D. Tanner, J. J. Chen, J. Org. Chem. 1989, 54, 3842–3846;
- 9cB. D. Naab, S. Guo, S. Olthof, E. G. B. Evans, P. Wei, G. L. Millhauser, A. Kahn, S. Barlow, S. R. Marder, Z. Bao, J. Am. Chem. Soc. 2013, 135, 15018–15025;
- 9dFor pioneering studies combining H atom transfer with electron transfer, see R. J. Kolt, D. D. M. Wayner, D. Griller, J. Org. Chem. 1989, 54, 4259–4260.
- 10J. R. Ames, M. A. Houghtaling, D. L. Terrian, T. P. Mitchell, Can. J. Chem. 1997, 75, 28.
- 11For a typical literature procedure see
- 11aT. Igarashi, E. Tayama, H. Iwamoto, E. Hasegawa, Tetrahedron Lett. 2013, 54, 6874–6877; This procedure was substantially modified to suit our needs, see supporting information;
- 11bSee supporting information, “Biphenyl 34—Preliminary Optimisation Studies of Reaction Conditions” pp. 7–9;
- 11cSee supporting information, “tert-Butyl-3-methylindoline-1-carboxylate 17—Optimisation for General Procedure A”, pp. 9–11;
- 11dSee supporting information, “Calculated Oxidation Potentials” p. 37.
- 12For alternative outcomes of reactions of imidazolium salts with NaBH4, see
- 12aS. Gardner, T. Kawamoto, D. P. Curran, J. Org. Chem. 2015, 80, 9794–9797;
- 12bE. F. Godefroi, J. Org. Chem. 1968, 33, 860–862.
- 13The aminal hydrogen atoms in 13 are hydridic. Consequently, the abstraction of one of these hydrogen atoms by a nucleophilic carbon centred radical is slow due to a mismatch in polarity. The thiol acts as a mediator in this step. The hydrogen abstraction from the thiol by a carbon centred radical is matched in polarity and fast. The thiyl radical that is generated is electrophilic and can efficiently abstract an aminal hydrogen atom. For further information on PRC see
- 13aB. P. Roberts, Chem. Soc. Rev. 1999, 28, 25–35;
- 13bH. S. Dang, M. R. J. Elsegood, K.-M. Kim, B. P. Roberts, J. Chem. Soc. Perkin Trans. 1 1999, 2061–2068;
- 13cA. J. Fielding, B. P. Roberts, Tetrahedron Lett. 2001, 42, 4061–4064.
- 14
- 14aX.-Q. Zhu, M.-T. Zhang, A. Yu, C.-H. Wang, J.-P. Cheng, J. Am. Chem. Soc. 2008, 130, 2501–2516;
- 14bJ. R. Aranzaes, M.-C. Daniel, D. Astruc, Can. J. Chem. 2006, 84, 288–299.
- 15G. N. Reddy, S. Giri, Phys. Chem. Chem. Phys. 2016, 18, 24356–24360.
- 16M. A. Syroeshkin, F. Kuriakose, E. A. Saverina, V. A. Timofeeva, M. P. Egorov, I. Alabugin, Angew. Chem. Int. Ed. 2019, 58, 5532–5550; Angew. Chem. 2019, 131, 5588–5607.