N-Heterocyclic Carbene Catalyzed (5+1) Annulations Exploiting a Vinyl Dianion Synthon Strategy
Xuan B. Nguyen
School of Chemistry, Monash University, Clayton, 3800 Victoria, Australia
Search for more papers by this authorDr. Yuji Nakano
School of Chemistry, Monash University, Clayton, 3800 Victoria, Australia
Search for more papers by this authorNisharnthi M. Duggan
School of Chemistry, Monash University, Clayton, 3800 Victoria, Australia
Search for more papers by this authorLydia Scott
School of Chemistry, Monash University, Clayton, 3800 Victoria, Australia
Search for more papers by this authorPriv.-Doz. Dr. Martin Breugst
Department für Chemie, Universität zu Köln, Greinstraße 4, 50939 Köln, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. David W. Lupton
School of Chemistry, Monash University, Clayton, 3800 Victoria, Australia
Search for more papers by this authorXuan B. Nguyen
School of Chemistry, Monash University, Clayton, 3800 Victoria, Australia
Search for more papers by this authorDr. Yuji Nakano
School of Chemistry, Monash University, Clayton, 3800 Victoria, Australia
Search for more papers by this authorNisharnthi M. Duggan
School of Chemistry, Monash University, Clayton, 3800 Victoria, Australia
Search for more papers by this authorLydia Scott
School of Chemistry, Monash University, Clayton, 3800 Victoria, Australia
Search for more papers by this authorPriv.-Doz. Dr. Martin Breugst
Department für Chemie, Universität zu Köln, Greinstraße 4, 50939 Köln, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. David W. Lupton
School of Chemistry, Monash University, Clayton, 3800 Victoria, Australia
Search for more papers by this authorGraphical Abstract
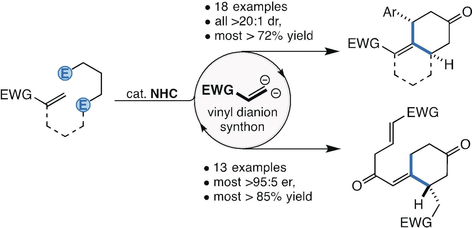
Inversion: Two C−C bonds are formed between three conjugate acceptors by N-heterocyclic carbene (NHC) catalyzed polarity inversion of α,β-unsaturated ketones and esters. Inter- and intramolecular (5+1) annulations are possible, exploiting an unusual vinyl dianion synthon strategy. The reaction provides access to mono- and bicyclic cyclohexanones. Mechanistic studies and derivatizations are also reported. EWG=electron-withdrawing group.
Abstract
Direct polarity inversion of conjugate acceptors provides a valuable entry to homoenolates. N-heterocyclic carbene (NHC) catalyzed reactions, in which β-unsubstituted conjugate acceptors undergo homoenolate formation and C−C bond formation twice, have been developed. Specifically, the all-carbon (5+1) annulations give a range of mono- and bicyclic cyclohexanones (31 examples). In the first family of annulations, β-unsubstituted acrylates tethered to a divinyl ketone undergo cycloisomerization, providing hexahydroindenes and tetralins. In the second, partially untethered substrates undergo an intermolecular (5+1) annulation involving dimerization followed by cycloisomerization. While enantioselectivity was not possible with the former, the latter proved viable, allowing cyclohexanones to be produced with high levels of enantiopurity (most >95:5 e.r.) and exclusive diastereoselectivity (>20:1 d.r.). Derivatizations and mechanistic studies are also reported.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201905475-sup-0001-misc_information.pdf16.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected recent reviews on conjugate addition reactions, see:
- 1aS. R. Harutyunyan, T. den Hartog, K. Geurts, A. J. Minnaard, B. L. Feringa, Chem. Rev. 2008, 108, 2824;
- 1bY. Zhang, W. Wang, Catal. Sci. Technol. 2012, 2, 42;
- 1cC. F. Nising, S. Bräse, Chem. Soc. Rev. 2012, 41, 988;
- 1dK. Zheng, X. Liu, X. Feng, Chem. Rev. 2018, 118, 7586;
- 1eFor a discussion of the discovery of the Michael addition: T. Tokoroyama, Eur. J. Org. Chem. 2010, 2009.
- 2For reviews on homoenolate chemistry, see:
- 2aD. Hoppe, Angew. Chem. Int. Ed. Engl. 1984, 23, 932–948; Angew. Chem. 1984, 96, 930–946; I. Kuwajima, E. Nakamura, Top. Curr. Chem. 1990, 155, 1;
- 2bD. Hoppe, T. Hense, Angew. Chem. Int. Ed. Engl. 1997, 36, 2282–2316; Angew. Chem. 1997, 109, 2376–2410;
- 2cH. Ahlbrecht, U. Beyer, Synthesis 1999, 365–390; O. G. Kulinkovich, Chem. Rev. 2003, 103, 2597;
- 2dD. Hoppe, Synthesis 2009, 43–55; I. Haym, M. A. Brimble, Org. Biomol. Chem. 2012, 10, 7649; L. R. Mills, S. A. L. Rousseaux, Eur. J. Org. Chem. 2019, 8–26; for recent examples see:
- 2eT. Hémery, B. Wibbeling, R. Fröhlich, D. Hoppe, Synthesis 2010, 329–342;
- 2fS. Kollmann, R. Fröhlich, D. Hoppe, Synthesis 2010, 749–756; L. R. Mills, L. M. B. Arbelaez, S. A. L. Rousseaux, J. Am. Chem. Soc. 2017, 139, 11357.
- 3For selected metal catalyzed approaches to homoenolates, see:
- 3aE. Nakamura, S. Aoki, K. Sekiya, H. Oshino, I. Kuwajima, J. Am. Chem. Soc. 1987, 109, 8056;
- 3bG. A. Molander, D. E. Petrillo, Org. Lett. 2008, 10, 1795;
- 3cD. C. Davis, K. L. Walker, C. Hu, R. N. Zare, R. M. Waymouth, M. Dai, J. Am. Chem. Soc. 2016, 138, 10693;
- 3dX. Zhou, S. Yu, L. Kong, X. Li, ACS Catal. 2016, 6, 647;
- 3eJ. Yang, Y. Shen, Y. J. Lim, N. Yoshikai, Chem. Sci. 2018, 9, 6928.
- 4
- 4aN. Takashina, C. C. Price, J. Am. Chem. Soc. 1962, 84, 489–491; For a review covering this work in some detail: H. Guo, Y. C. Fan, Z. Sun, Y. Wu, O. Kwon, Chem. Rev. 2018, 118, 10049; For selected related chemistry see:
- 4bC. D. Hall, N. Lowther, B. R. Tweedy, A. C. Hall, G. Shaw, J. Chem. Soc. Perkin Trans. 2 1998, 2047–2054;
- 4cS. N. Khong, Y. S. Tran, O. Kwon, Tetrahedron 2010, 66, 4760–4768;
- 4dX.-B. Wang, Y. Saga, R. Shen, H. Fujino, M. Goto, L.-B. Han, RSC Adv. 2012, 2, 5935–5937;
- 4eS. Takizawa, K. Kishi, Y. Yoshida, S. Mader, F. A. Arteaga, S. Lee, M. Hoshino, M. Rueping, M. Fujita, H. Sasai, Angew. Chem. Int. Ed. 2015, 54, 15511–15515; Angew. Chem. 2015, 127, 15731–15735; For phospha-Michael chemistry, see:
- 4fD. Enders, A. Saint-Dizier, M.-I. Lannou, A. Lenzen, Eur. J. Org. Chem. 2006, 26–49.
- 5
- 5aY. Nakano, D. W. Lupton, Angew. Chem. Int. Ed. 2016, 55, 3135–3139; Angew. Chem. 2016, 128, 3187–3191;
- 5bL. Scott, Y. Nakano, C. Zhang, D. W. Lupton, Angew. Chem. Int. Ed. 2018, 57, 10299; Angew. Chem. 2018, 130, 10456.
- 6J. Ametovski, U. Dutta, L. Burchill, D. Maiti, D. W. Lupton, J. F. Hooper, Chem. Commun. 2017, 53, 13071.
- 7For general NHC catalysis see:
- 7aD. Enders, O. Niemeier, A. Henseler, Chem. Rev. 2007, 107, 5606–5655;
- 7bM. N. Hopkinson, C. Richter, M. Schedler, F. Glorius, Nature 2014, 510, 485–496;
- 7cD. M. Flanigan, F. Romanov-Michailidis, N. A. White, T. Rovis, Chem. Rev. 2015, 115, 9307–9387; for acyl azolium enolates see:
- 7dJ. Douglas, G. Churchill, A. D. Smith, Synthesis 2012, 44, 2295–2309; for cascade catalysis:
- 7eA. Grossmann, D. Enders, Angew. Chem. Int. Ed. 2012, 51, 314–325; Angew. Chem. 2012, 124, 320–332; for acyl anion free catalysis:
- 7fS. J. Ryan, L. Candish, D. W. Lupton, Chem. Soc. Rev. 2013, 42, 4906–4917; for acyl azolium catalysis:
- 7gS. De Sarkar, A. Biswas, R. C. Samanta, A. Studer, Chem. Eur. J. 2013, 19, 4664–4678;
- 7hC. Zhang, J. F. Hooper, D. W. Lupton, ACS Catal. 2017, 7, 2583–2596; S. Mondal, S. R. Yetra, S. Mukherjee, A. T. Biju, Acc. Chem. Res. 2019, 52, 425–436; for cooperative catalysis:
- 7iM. H. Wang, K. A. Scheidt, Angew. Chem. Int. Ed. 2016, 55, 14912–14922; Angew. Chem. 2016, 128, 15134–15145.
- 8For homoenolate by NHC 1,4-addition, see:
- 8aC. Fischer, S. W. Smith, D. A. Powell, G. C. Fu, J. Am. Chem. Soc. 2006, 128, 1472–1473; for a review discussing NHC 1,4-addition reactions, see:
- 8bX.-Y. Chen, S. Ye, Org. Biomol. Chem. 2013, 11, 7991–7998; for polymerization by 1,4-addition of NHCs, see:
- 8cY. Zhang, E. Y.-X. Chen, Angew. Chem. Int. Ed. 2012, 51, 2465–2469; Angew. Chem. 2012, 124, 2515–2519;
- 8dM. Hong, E. Y.-X. Chen, Angew. Chem. Int. Ed. 2014, 53, 11900–11906; Angew. Chem. 2014, 126, 12094–12100; for a stoichiometric example, see:
- 8eW. N. Ottou, D. Bourichon, J. Vignolle, A.-L. Wirotius, F. Robert, Y. Landais, J.-M. Sotiropoulos, K. Miqueu, D. Taton, Chem. Eur. J. 2015, 21, 9447–9453.
- 9The compound 4 is a type of deoxy-Breslow intermediate, a name used to describe compounds with a formal double bond from the NHC to a carbon center lacking an oxygen substituent. For other examples, see:
- 9aD. Enders, K. Breuer, G. Raabe, J. Runsink, J. H. Teles, J.-P. Melder, K. Ebel, S. Brode, Angew. Chem. Int. Ed. Engl. 1995, 34, 1021–1023; Angew. Chem. 1995, 107, 1119–1122;
- 9bC. E. I. Knappke, J.-M. Neudörfl, A. J. von Wangelin, Org. Biomol. Chem. 2010, 8, 1695–1705;
- 9cC. E. I. Knappke, A. J. Arduengo III, H. Jiao, J. Haijun, J.-M. Neudörfl, A. J. von Wangelin, Synthesis 2011, 3784–3795;
- 9dR. N. Reddi, P. K. Prasad, A. Sudalai, Angew. Chem. Int. Ed. 2015, 54, 14150–14153; Angew. Chem. 2015, 127, 14356–14359;
- 9eM. Schedler, N. E. Wurz, C. G. Daniliuc, F. Glorius, Org. Lett. 2014, 16, 3134–3137;
- 9fA. Bhunia, S. Thorat, R. G. Gonnade, A. T. Biju, Chem. Commun. 2015, 51, 13690–13693; for properties of see:
- 9gA. Berkessel, S. Elfert, Adv. Synth. Catal. 2014, 356, 571–578;
- 9hB. Maji, M. Horn, H. Mayr, Angew. Chem. Int. Ed. 2012, 51, 6231–6235; Angew. Chem. 2012, 124, 6335–6339;
- 9iB. Maji, H. Mayr, Angew. Chem. Int. Ed. 2012, 51, 10408–10412; Angew. Chem. 2012, 124, 10554–10558.
- 10For discovery of enal-derived homoenolates, see:
- 10aC. Burstein, F. Glorius, Angew. Chem. Int. Ed. 2004, 43, 6205–6208; Angew. Chem. 2004, 116, 6331–6334;
- 10bK. Y.-K. Chow, J. W. Bode, J. Am. Chem. Soc. 2004, 126, 8126–8127; For selected examples:
- 10cN. T. Reynolds, J. R. de Alaniz, T. Rovis, J. Am. Chem. Soc. 2004, 126, 9518–9519;
- 10dS. S. Sohn, E. L. Rosen, J. W. Bode, J. Am. Chem. Soc. 2004, 126, 14370–14371;
- 10eA. Chan, K. A. Scheidt, Org. Lett. 2005, 7, 905–908;
- 10fN. T. Reynolds, T. Rovis, J. Am. Chem. Soc. 2005, 127, 16406–16407; for reviews see:
- 10gV. Nair, R. S. Menon, A. T. Biju, C. R. Sinu, R. R. Paul, A. Jose, V. Sreekumar, Chem. Soc. Rev. 2011, 40, 5336–5346;
- 10hR. S. Menon, A. T. Biju, V. Nair, Beilstein J. Org. Chem. 2016, 12, 444–461.
- 11
- 11aS.-i. Matsuoka, Y. Ota, A. Washio, A. Katada, K. Ichioka, K. Takagi, M. Suzuki, Org. Lett. 2011, 13, 3722–3725;
- 11bS.-i. Matsuoka, Y. Tochigi, K. Takagi, M. Suzuki, Tetrahedron 2012, 68, 9836–9841;
- 11cS.-i. Matsuoka, S. Namera, A. Washio, K. Takagi, M. Suzuki, Org. Lett. 2013, 15, 5916–5919;
- 11dT. Kato, Y. Ota, S.-i. Matsuoka, K. Takagi, M. Suzuki, J. Org. Chem. 2013, 78, 8739–8747;
- 11eT. Kato, S.-i. Matsuoka, M. Suzuki, J. Org. Chem. 2014, 79, 4484–4494;
- 11fS.-i. Matsuoka, M. Nakazawa, M. Suzuki, Bull. Chem. Soc. Jpn. 2015, 88, 1093–1099;
- 11gS.-i. Matsuoka, N. Awano, M. Nakazawa, M. Suzuki, Tetrahedron Lett. 2016, 57, 5707–5711.
- 12A. T. Biju, M. Padmanaban, N. E. Wurz, F. Glorius, Angew. Chem. Int. Ed. 2011, 50, 8412–8415; Angew. Chem. 2011, 123, 8562–8565.
- 13O.-a. Rajachan, M. Paul, V. R. Yatham, J.-M. Neudörfl, K. Kanokmedhakul, S. Kanokmedhakul, A. Berkessel, Tetrahedron Lett. 2015, 56, 6537–6540.
- 14For the impact of N-substituent on nucleophilcity of NHCs, see:
- 14aA. Levens, F. An, M. Breugst, H. Mayr, D. W. Lupton, Org. Lett. 2016, 18, 3566–3569;
- 14bM. S. Kerr, J. Read de Alaniz, T. Rovis, J. Am. Chem. Soc. 2002, 124, 10298–10299;
- 14cJ. Read de Alaniz, T. Rovis, J. Am. Chem. Soc. 2005, 127, 6284–6289;
- 14dC. J. Collett, R. S. Massey, O. R. Maguire, A. S. Batsanov, A. C. O'Donoghue, A. D. Smith, Chem. Sci. 2013, 4, 1514–1522.
- 15Related adducts have been proposed previously, see for example: R. C. Johnston, D. T. Cohen, C. C. Eichman, K. A. Scheidt, P. H.-Y. Cheong, Chem. Sci. 2014, 5, 1974–1982.
- 16For a review see:
- 16aD. J. Faulkner, Synthesis 1971, 175–189;
- 16bA. B. Flynn, W. W. Ogilvie, Chem. Rev. 2007, 107, 4698–4745;
- 16cE.-i. Negishi, Z. Huang, G. Wang, S. Mohan, C. Wang, H. Hattori, Acc. Chem. Res. 2008, 41, 1474–1485; and for examples, see:
- 16dD. A. Engel, G. B. Dudley, Org. Lett. 2006, 8, 4027–4029;
- 16eC. J. Rieder, K. J. Winberg, F. G. West, J. Am. Chem. Soc. 2009, 131, 7504–7505;
- 16fT. T. Nguyen, M. J. Koh, T. J. Mann, R. R. Schrock, A. H. Hoveyda, Nature 2017, 552, 347–354, and references therein.
- 17Rates calculated at 10 and 20 mol % C3. See the Supporting Information for details.
- 18For the impact of ester tethers in cyclization reactions, see:
- 18aC. Kammerer, G. Prestat, D. Madec, G. Poli, Acc. Chem. Res. 2014, 47, 3439–3447; for the effects of ring size on cyclizations see:
- 18bH. Ishibashi, Chem. Rec. 2006, 6, 23–31; for Thorpe–Ingold effects:
- 18cM. E. Jung, G. Piizzi, Chem. Rev. 2005, 105, 1735–1766.
- 19The CD spectra of 28 h were calculated at the TD-B2PLYP-D3/def2-TZVP/SMD(CH3CN)//B3LYP-D3BJ/6–31+G(d,p)/SMD(CH3CN) level of theory. For details see the Supporting Information.
- 20
- 20aM. R. Colsman, M. D. Noirot, M. M. Miller, O. P. Anderson, S. H. Strauss, J. Am. Chem. Soc. 1988, 110, 6886–6888;
- 20bM. R. Colsman, T. D. Newbound, L. J. Marshall, M. D. Noirot, M. M. Miller, G. P. Wulfsberg, J. S. Frye, O. P. Anderson, S. H. Strauss, J. Am. Chem. Soc. 1990, 112, 2349–2362;
- 20cP. K. Hurlburt, O. P. Anderson, S. H. Strauss, Can. J. Chem. 1992, 70, 726–731;
- 20dT. G. Levitskaia, J. C. Bryan, R. A. Sachleben, J. D. Lamb, B. A. Moyer, J. Am. Chem. Soc. 2000, 122, 554–562;
- 20eS. Irvine, W. J. Kerr, A. R. McPherson, C. M. Pearson, Tetrahedron 2008, 64, 926–935, and references therein.
- 21For NHC generation see the Supporting Information. For other reactions with salt sensitivity, see:
- 21aS. J. Ryan, L. Candish, D. W. Lupton, J. Am. Chem. Soc. 2011, 133, 4694–4697;
- 21bA. Levens, A. Ametovski, D. W. Lupton, Angew. Chem. Int. Ed. 2016, 55, 16136–16140; Angew. Chem. 2016, 128, 16370–16374.