Dinitrogen-Molybdenum Complex Induces Dinitrogen Cleavage by One-Electron Oxidation
Dr. Akira Katayama
Department of Cooperative Major in Nanopharmaceutical Sciences, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa, Nagoya, 466-8555 Japan
Search for more papers by this authorProf. Dr. Takehiro Ohta
Picobiology Institute, Graduate School of Life Science, University of Hyogo, RSC-UH LP Center, Hyogo, 679-5148 Japan
Present address: Department of Applied Chemistry, Faculty of Engineering, Sanyo-Onoda City University, Sanyo-Onoda, Yamaguchi, 756-0884 Japan
Search for more papers by this authorDr. Yuko Wasada-Tsutsui
Department of Cooperative Major in Nanopharmaceutical Sciences, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa, Nagoya, 466-8555 Japan
Search for more papers by this authorProf. Dr. Tomohiko Inomata
Department of Life Science and Applied Chemistry, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa, Nagoya, 466-8555 Japan
Search for more papers by this authorProf. Dr. Tomohiro Ozawa
Department of Cooperative Major in Nanopharmaceutical Sciences, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa, Nagoya, 466-8555 Japan
Search for more papers by this authorProf. Dr. Takashi Ogura
Picobiology Institute, Graduate School of Life Science, University of Hyogo, RSC-UH LP Center, Hyogo, 679-5148 Japan
Deceased
Search for more papers by this authorCorresponding Author
Prof. Dr. Hideki Masuda
Department of Life Science and Applied Chemistry, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa, Nagoya, 466-8555 Japan
Search for more papers by this authorDr. Akira Katayama
Department of Cooperative Major in Nanopharmaceutical Sciences, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa, Nagoya, 466-8555 Japan
Search for more papers by this authorProf. Dr. Takehiro Ohta
Picobiology Institute, Graduate School of Life Science, University of Hyogo, RSC-UH LP Center, Hyogo, 679-5148 Japan
Present address: Department of Applied Chemistry, Faculty of Engineering, Sanyo-Onoda City University, Sanyo-Onoda, Yamaguchi, 756-0884 Japan
Search for more papers by this authorDr. Yuko Wasada-Tsutsui
Department of Cooperative Major in Nanopharmaceutical Sciences, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa, Nagoya, 466-8555 Japan
Search for more papers by this authorProf. Dr. Tomohiko Inomata
Department of Life Science and Applied Chemistry, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa, Nagoya, 466-8555 Japan
Search for more papers by this authorProf. Dr. Tomohiro Ozawa
Department of Cooperative Major in Nanopharmaceutical Sciences, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa, Nagoya, 466-8555 Japan
Search for more papers by this authorProf. Dr. Takashi Ogura
Picobiology Institute, Graduate School of Life Science, University of Hyogo, RSC-UH LP Center, Hyogo, 679-5148 Japan
Deceased
Search for more papers by this authorCorresponding Author
Prof. Dr. Hideki Masuda
Department of Life Science and Applied Chemistry, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa, Nagoya, 466-8555 Japan
Search for more papers by this authorGraphical Abstract
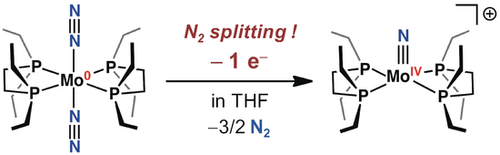
Time to split: N2 cleavage in a one-electron oxidation reaction of trans-[Mo(depe)2(N2)2], a simple molybdenum complex supported by bidentate phosphine ligands, is studied. The one-electron chemical and electrochemical oxidation reaction of Mo0 to MoI produces a MoIV terminal nitride complex via formation of MoII−N=N−MoII structures, which undergo cleavage of the bridging N2 molecule.
Abstract
Reported here is the N2 cleavage of a one-electron oxidation reaction using trans-[Mo(depe)2(N2)2] (1) (depe=Et2PCH2CH2PEt2), which is a classical molybdenum(0)-dinitrogen complex supported by two bidentate phosphine ligands. The molybdenum(IV) terminal nitride complex [Mo(depe)2N][BArf4] (2) (BArf4=B(3,5-(CF3)2C6H3)4) is synthesized by the one-electron oxidation of 1 upon addition of a mild oxidant, [Cp2Fe][BArf4] (Cp=C5H5), and proceeds by N2 cleavage from a MoII-N=N-MoII structure. In addition, the electrochemical oxidation reaction for 1 also cleaved the N2 ligand to give 2. The dimeric Mo complex with a bridging N2 is detected by in situ resonance Raman and in situ UV-vis spectroscopies during the electrochemical oxidation reaction for 1. Density-functional theory (DFT) calculations reveal that the unstable monomeric oxidized MoI species is converted into 2 via the dimeric structure involving a zigzag transition state.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201905299-sup-0001-misc_information.pdf40.7 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. J. Burford, M. D. Fryzuk, Nat. Rev. Chem. 2017, 1, 1–13;
- 1bP. Bhattacharya, D. E. Prokopchuk, M. T. Mock, Coord. Chem. Rev. 2017, 334, 67–83;
- 1cY. Nishibayashi, Inorg. Chem. 2015, 54, 9234–9247;
- 1dP. J. Hill, L. R. Doyle, A. D. Crawford, W. K. Myers, A. E. Ashley, J. Am. Chem. Soc. 2016, 138, 13521–13524;
- 1eT. M. Buscagan, P. H. Oyala, J. C. Peters, Angew. Chem. Int. Ed. 2017, 56, 6921–6926; Angew. Chem. 2017, 129, 7025–7030;
- 1fL. A. Wickramasinghe, T. Ogawa, R. R. Schrock, P. Müller, J. Am. Chem. Soc. 2017, 139, 9132–9135;
- 1gR. Araake, K. Sakadani, M. Tada, Y. Sakai, Y. Ohki, J. Am. Chem. Soc. 2017, 139, 5596–5606;
- 1hP. Bhattacharya, Z. M. Heiden, E. S. Wiedner, S. Raugei, N. A. Piro, W. S. Kassel, R. M. Bullock, M. T. Mock, J. Am. Chem. Soc. 2017, 139, 2916–2919;
- 1iI. Klopsch, E. Y. Yuzik-Klimova, S. Schneider, Top. Organomet. Chem. 2017, 60, 71–112;
- 1jY. Nishibayashi, Dalton Trans. 2018, 47, 11290–11297.
- 2
- 2aV. K. Shah, W. J. Brill, Proc. Natl. Acad. Sci. USA 1977, 74, 3249–3253;
- 2bT. Spatzal, M. Aksoyoglu, L. Zhang, S. L. A. Andrade, E. Schleicher, S. Weber, D. C. Rees, O. Einsle, Science 2011, 334, 940;
- 2cK. M. Lancaster, M. Roemelt, P. Ettenhuber, Y. Hu, M. W. Ribbe, F. Neese, U. Bergmann, S. DeBeer, Science 2011, 334, 974–977.
- 3Reviews:
- 3aM. Appl, Ammonia: Principles and Industrial Practice, Wiley-VCH, Weinheim, 1999;
10.1002/9783527613885 Google Scholar
- 3bG. Ertl, Angew. Chem. Int. Ed. 2008, 47, 3524; Angew. Chem. 2008, 120, 3578.
- 4
- 4aM. Hidai, Y. Mizobe, Chem. Rev. 1995, 95, 1115–1133;
- 4bJ. Chatt, A. J. Pearman, R. L. Richards, J. Chem. Soc. Dalton Trans. 1977, 1852–1860.
- 5J. Chatt, R. L. Richards, J. Organomet. Chem. 1982, 239, 65–77.
- 6D. V. Yandulov, R. R. Schrock, Science 2003, 301, 76–78.
- 7
- 7aK. Arashiba, Y. Miyake, Y. Nishibayashi, Nat. Chem. 2011, 3, 120–125;
- 7bK. Arashiba, E. Kinoshita, S. Kuriyama, A. Eizawa, K. Nakajima, H. Tanaka, K. Yoshizawa, Y. Nishibayashi, J. Am. Chem. Soc. 2015, 137, 5666–5669;
- 7cA. Eizawa, K. Arashiba, H. Tanaka, S. Kuriyama, Y. Matsuo, K. Nakajima, K. Yoshizawa, Y. Nishibayashi, Nat. Commun. 2017, 8, 14874;
- 7dS. Kuriyama, K. Arashiba, K. Nakajima, H. Tanaka, N. Kamaru, K. Yoshizawa, Y. Nishibayashi, J. Am. Chem. Soc. 2014, 136, 9719–9731;
- 7eY. Ashida, K. Arashiba, K. Nakajima, Y. Nishibayashi, Nature 2019, 568, 536–540.
- 8
- 8aC. E. Laplaza, C. C. Cummins, Science 1995, 268, 861–863;
- 8bC. E. Laplaza, M. J. A. Johnson, J. C. Peters, A. L. Odom, E. Kim, C. C. Cummins, G. N. George, I. J. Pickering, J. Am. Chem. Soc. 1996, 118, 8623–8638;
- 8cJ. J. Curley, T. R. Cook, S. Y. Reece, P. Müller, C. C. Cummins, J. Am. Chem. Soc. 2008, 130, 9394–9405.
- 9C. Rebreyend, B. de Bruin, Angew. Chem. Int. Ed. 2015, 54, 42–44; Angew. Chem. 2015, 127, 42–44.
- 10M. J. Bezdek, P. J. Chirik, Angew. Chem. Int. Ed. 2016, 55, 7892–7896; Angew. Chem. 2016, 128, 8022–8026.
- 11J. S. Figueroa, N. A. Piro, J. Am. Chem. Soc. 2006, 128, 940–950.
- 12H. Kunkely, A. Vogler, Angew. Chem. Int. Ed. 2010, 49, 1591–1593; Angew. Chem. 2010, 122, 1636–1638.
- 13M. M. Rodriguez, E. Bill, W. W. Brennessel, P. L. Holland, Science 2011, 334, 780–783.
- 14B. Askevold, J. T. Nieto, S. Tussupbayev, M. Diefenbach, E. Herdtweck, M. C. Holthausen, S. Schneider, Nat. Chem. 2011, 3, 532–537.
- 15T. Shima, S. Hu, G. Luo, X. Kang, Y. Luo, Z. Hou, Science 2013, 340, 1549–1552.
- 16I. Klopsch, M. Finger, C. Würtele, B. Milde, D. B. Werz, S. Schneider, J. Am. Chem. Soc. 2014, 136, 6881–6883.
- 17A. J. Keane, W. S. Farrell, B. L. Yonke, P. Y. Zavalij, L. R. Sita, Angew. Chem. Int. Ed. 2015, 54, 10220–10224; Angew. Chem. 2015, 127, 10358–10362.
- 18
- 18aI. Klopsch, M. Kinauer, M. Finger, C. Würtele, S. Schneider, Angew. Chem. Int. Ed. 2016, 55, 4786–4789; Angew. Chem. 2016, 128, 4864–4867;
- 18bF. Schendzielorz, M. Finger, J. Abbenseth, C. Würtele, V. Krewald, S. Schneider, Angew. Chem. Int. Ed. 2019, 58, 830–834; Angew. Chem. 2019, 131, 840–844.
- 19M. M. Guru, T. Shima, Z. Hou, Angew. Chem. Int. Ed. 2016, 55, 12316–12320; Angew. Chem. 2016, 128, 12504–12508.
- 20F. S. Schendzielorz, M. Finger, C. Volkmann, C. Würtele, S. Schneider, Angew. Chem. Int. Ed. 2016, 55, 11417–11420; Angew. Chem. 2016, 128, 11589–11592.
- 21G. A. Silantyev, M. Förster, B. Schluschaß, J. Abbenseth, C. Würtele, C. Volkmann, M. C. Holthausen, S. Schneider, Angew. Chem. Int. Ed. 2017, 56, 5872–5876; Angew. Chem. 2017, 129, 5966–5970.
- 22
- 22aY. Nakanishi, Y. Ishida, H. Kawaguchi, Angew. Chem. Int. Ed. 2017, 56, 9193–9197; Angew. Chem. 2017, 129, 9321—9325;
- 22bF. Akagi, T. Matsuo, H. Kawaguchi, Angew. Chem. Int. Ed. 2007, 46, 8778–8781; Angew. Chem. 2007, 119, 8934—8937.
- 23M. Hirotsu, P. P. Fontaine, A. Epshteyn, P. Y. Zavalij, L. R. Sita, J. Am. Chem. Soc. 2007, 129, 9284–9285.
- 24A. J. Keane, B. J. Yonke, M. Hirotsu, P. Y. Zavalij, L. R. Sita, J. Am. Chem. Soc. 2014, 136, 9906–9909.
- 25K. Searles, P. J. Carroll, C. Chen, M. Pink, D. J. Mindiola, Chem. Commun. 2015, 51, 3526–3528.
- 26T. Miyazaki, H. Tanaka, Y. Tanabe, M. Yuki, K. Nakajima, K. Yoshizawa, Y. Nishibayashi, Angew. Chem. Int. Ed. 2014, 53, 11488–11492; Angew. Chem. 2014, 126, 11672–11676.
- 27B. M. Lindley, R. S. van Alten, M. Finger, F. Schendzielorz, C. Wurtelete, A. J. M. Miller, I. Siewert, S. Schneider, J. Am. Chem. Soc. 2018, 140, 7922–7935.
- 28T. J. Hebden, R. R. Schrock, M. K. Takase, P. Müller, Chem. Commun. 2012, 48, 1851–1853.
- 29Q. Liao, A. Cavaillé, N. Saffon-Merceron, N. Mézailles, Angew. Chem. Int. Ed. 2016, 55, 11212–11216; Angew. Chem. 2016, 128, 11378–11382.
- 30K. Arashiba, A. Eizawa, H. Tanaka, K. Nakajima, K. Yoshizawa, Y. Nishibayashi, Bull. Chem. Soc. Jpn. 2017, 90, 1111–1118.
- 31J. Cugny, H. W. Schmalle, T. Fox, O. Blacque, M. Alfonso, H. Berke, Eur. J. Inorg. Chem. 2006, 540–552.
- 32
- 32aJ. R. Durig, M. G. Griffin, R. W. MacNamee, J. Raman Spectrosc. 1975, 3, 133–141;
- 32bN. C. Craig, I. W. Levin, J. Chem. Phys. 1979, 71, 400–407.