Reversing Chemoselectivity: Simultaneous Positive and Negative Catalysis by Chemically Equivalent Rims of a Cucurbit[7]uril Host
Dr. Nazar Rad
Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland
Search for more papers by this authorDr. Oksana Danylyuk
Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland
Search for more papers by this authorCorresponding Author
Prof. Volodymyr Sashuk
Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland
Search for more papers by this authorDr. Nazar Rad
Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland
Search for more papers by this authorDr. Oksana Danylyuk
Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland
Search for more papers by this authorCorresponding Author
Prof. Volodymyr Sashuk
Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland
Search for more papers by this authorGraphical Abstract
Abstract
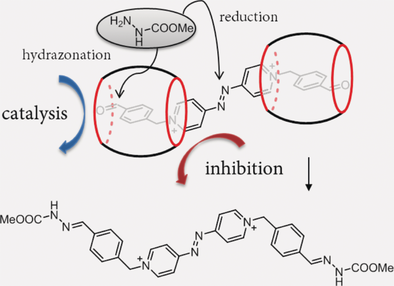
Enzyme catalysis has always been an inspiration and an unattainable goal for chemists due to features such as high specificity, selectivity, and efficiency. Here, we disclose a feature neither common in enzymes nor ever described for enzyme mimics, but one that could prove crucial for the catalytic performance of the latter, namely the ability to catalyze and inhibit two different reactions at the same time. Remarkably, this can be realized by two identical, spatially resolved catalytic sites. In the future, such a synchronized catalyst action could be used not only for controlling chemoselectivity, as in the present case, but also for regulating other types of chemical reactivity.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201905027-sup-0001-misc_information.pdf3.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aL. Pauling, Chem. Eng. News 1946, 24, 1375–1377;
- 1bL. Pauling, Nature 1948, 161, 707.
- 2
- 2aB. Vögeli, T. J. Erb, Curr. Opin. Chem. Biol. 2018, 47, 94–100;
- 2bJ. Rétey, Angew. Chem. Int. Ed. Engl. 1990, 29, 355–361; Angew. Chem. 1990, 102, 373–379.
- 3
- 3aE. Kuah, S. Toh, J. Yee, Q. Ma, Z. Gao, Chem. Eur. J. 2016, 22, 8404–8430;
- 3bM. Raynal, P. Ballester, A. Vidal-Ferran, P. W. N. M. van Leeuwen, Chem. Soc. Rev. 2014, 43, 1734–1787.
- 4
- 4aR. Breslow, S. D. Dong, Chem. Rev. 1998, 98, 1997–2012;
- 4b“Calixarenes in Organo and Biomimetic Catalysis”: M. Yilmaz, S. Sayin, in Calixarenes and Beyond (Eds.: ), Springer International Publishing, Cham, 2016, pp. 719–742.
10.1007/978-3-319-31867-7_27 Google Scholar
- 5
- 5aC. J. Brown, F. D. Toste, R. G. Bergman, K. N. Raymond, Chem. Rev. 2015, 115, 3012–3035;
- 5bS. Zarra, D. M. Wood, D. A. Roberts, J. R. Nitschke, Chem. Soc. Rev. 2015, 44, 419–432.
- 6
- 6aQ. Zhang, L. Catti, K. Tiefenbacher, Acc. Chem. Res. 2018, 51, 2107–2114;
- 6bY. Zhu, J. Rebek, Jr., Y. Yu, Chem. Commun. 2019, 55, 3573–3577.
- 7
- 7aJ. Wu, X. Wang, Q. Wang, Z. Lou, S. Li, Y. Zhu, L. Qin, H. Wei, Chem. Soc. Rev. 2019, 48, 1004–1076;
- 7bY. Huang, J. Ren, X. Qu, Chem. Rev. 2019, 119, 4357–4412.
- 8
- 8aK. I. Assaf, W. M. Nau, Chem. Soc. Rev. 2015, 44, 394–418;
- 8bB. C. Pemberton, R. Raghunathan, S. Volla, J. Sivaguru, Chem. Eur. J. 2012, 18, 12178–12190;
- 8cL. Zheng, S. Sonzini, M. Ambarwati, E. Rosta, O. A. Scherman, A. Herrmann, Angew. Chem. Int. Ed. 2015, 54, 13007–13011; Angew. Chem. 2015, 127, 13199–13203;
- 8dA. Palma, M. Artelsmair, G. Wu, X. Lu, S. J. Barrow, N. Uddin, E. Rosta, E. Masson, O. A. Scherman, Angew. Chem. Int. Ed. 2017, 56, 15688–15692; Angew. Chem. 2017, 129, 15894–15898.
- 9
- 9aF. Tian, D. Jiao, F. Biedermann, O. A. Scherman, Nat. Commun. 2012, 3, 1207;
- 9bH.-B. Cheng, Y.-M. Zhang, C. Xu, Y. Liu, Sci. Rep. 2014, 4, 4210;
- 9cM. Baroncini, C. Gao, V. Carboni, A. Credi, E. Previtera, M. Semeraro, M. Venturi, S. Silvi, Chem. Eur. J. 2014, 20, 10737–10744;
- 9dJ. Wu, L. Isaacs, Chem. Eur. J. 2009, 15, 11675–11680;
- 9eW. Wang, X. Wang, J. Cao, J. Liu, B. Qi, X. Zhou, S. Zhang, D. Gabel, W. M. Nau, K. I. Assaf, H. Zhang, Chem. Commun. 2018, 54, 2098–2101;
- 9fH. Huang, A. Juan, N. Katsonis, J. Huskens, Tetrahedron 2017, 73, 4913–4917.
- 10H. Mutlu, C. M. Geiselhart, C. Barner-Kowollik, Mater. Horiz. 2018, 5, 162–183.
- 11S. J. Barrow, S. Kasera, M. J. Rowland, J. del Barrio, O. A. Scherman, Chem. Rev. 2015, 115, 12320–12406.
- 12
- 12aV. Sashuk, H. Butkiewicz, M. Fialkowski, O. Danylyuk, Chem. Commun. 2016, 52, 4191–4194;
- 12bC. Klöck, R. N. Dsouza, W. M. Nau, Org. Lett. 2009, 11, 2595–2598.
- 13J. Kalia, R. T. Raines, Angew. Chem. Int. Ed. 2008, 47, 7523–7526; Angew. Chem. 2008, 120, 7633–7636.
- 14
- 14aL. Scorsin, J. A. Roehrs, R. R. Campedelli, G. F. Caramori, A. O. Ortolan, R. L. T. Parreira, H. D. Fiedler, A. Acuña, L. García-Río, F. Nome, ACS Catal. 2018, 8, 12067–12079;
- 14bN. Basilio, L. García-Río, J. A. Moreira, M. Pessêgo, J. Org. Chem. 2010, 75, 848–855;
- 14cW. Gong, J. Ma, Z. Zhao, F. Gao, F. Liang, H. Zhang, S. Liu, J. Org. Chem. 2017, 82, 3298–3301.
- 15D. H. Macartney, Isr. J. Chem. 2018, 58, 230–243.
- 16H. Cong, C.-R. Li, S.-F. Xue, Z. Tao, Q.-J. Zhu, G. Wei, Org. Biomol. Chem. 2011, 9, 1041–1046.
- 17S. Senler, B. Cheng, A. E. Kaifer, Org. Lett. 2014, 16, 5834–5837.
- 18T. Ogoshi, S. Kanai, S. Fujinami, T.-a. Yamagishi, Y. Nakamoto, J. Am. Chem. Soc. 2008, 130, 5022–5023.
- 19
- 19aS. Kosiorek, B. Rosa, T. Boinski, H. Butkiewicz, M. P. Szymanski, O. Danylyuk, A. Szumna, V. Sashuk, Chem. Commun. 2017, 53, 13320–13323;
- 19bS. Kosiorek, H. Butkiewicz, O. Danylyuk, V. Sashuk, Chem. Commun. 2018, 54, 6316–6319.
- 20M. Szewczyk, G. Sobczak, V. Sashuk, ACS Catal. 2018, 8, 2810–2814.