Stable Cross-Conjugated Tetrathiophene Diradical
Dr. Cheng Zhang
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Organic Solids, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 China
These authors contributed equally to this work.
co-first author
Search for more papers by this authorSamara Medina Rivero
Department of Physical Chemistry, University of Málaga, Andalucia-Tech, Campus de Teatinos s/n, 29071 Málaga, Spain
These authors contributed equally to this work.
co-first author
Search for more papers by this authorWuyue Liu
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Organic Solids, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorDr. David Casanova
Donostia, International Physics Center (DIPC) & IKERBASQUE—, Basque Foundation for Science, 20018 Donostia- San Sebastián, Euskadi, Spain
Search for more papers by this authorCorresponding Author
Prof. Xiaozhang Zhu
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Organic Solids, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorCorresponding Author
Prof. Juan Casado
Department of Physical Chemistry, University of Málaga, Andalucia-Tech, Campus de Teatinos s/n, 29071 Málaga, Spain
Search for more papers by this authorDr. Cheng Zhang
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Organic Solids, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 China
These authors contributed equally to this work.
co-first author
Search for more papers by this authorSamara Medina Rivero
Department of Physical Chemistry, University of Málaga, Andalucia-Tech, Campus de Teatinos s/n, 29071 Málaga, Spain
These authors contributed equally to this work.
co-first author
Search for more papers by this authorWuyue Liu
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Organic Solids, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorDr. David Casanova
Donostia, International Physics Center (DIPC) & IKERBASQUE—, Basque Foundation for Science, 20018 Donostia- San Sebastián, Euskadi, Spain
Search for more papers by this authorCorresponding Author
Prof. Xiaozhang Zhu
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Organic Solids, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190 P. R. China
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorCorresponding Author
Prof. Juan Casado
Department of Physical Chemistry, University of Málaga, Andalucia-Tech, Campus de Teatinos s/n, 29071 Málaga, Spain
Search for more papers by this authorThese authors contributed equally to this work.
co-first author
Graphical Abstract
Abstract
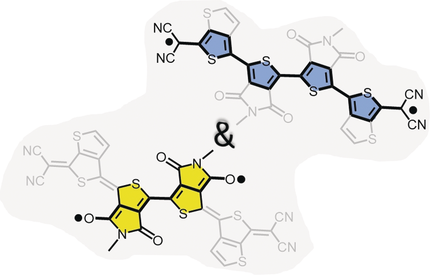
A tetracyano quinoidal tetrathiophene, having a central bi(thieno[3,4-c]pyrrole-4,6-dione) acceptor, has been studied. The recovered aromaticity of the thiophenes produces a diradical species with cross-conjugation between the inter-dicyano and inter-dione acceptor paths. A diradical character of y0=0.61 and a singlet–triplet gap of −2.76 kcal mol−1 were determined. Competition between the two cross-conjugated paths enhances the disjointed character of the SOMOs and results in the confinement of the diradical to the molecular center, enabling a thermodynamic diradical stabilization featuring a half-life of 262 hours. Cross-conjugation effects have been also addressed in the anionic species (up to a radical trianion).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201904153-sup-0001-misc_information.pdf2.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a Diradicals (Ed.: ), Wiley, New York, 1982;
- 1bM. Abe, Chem. Rev. 2013, 113, 7011–7088.
- 2
- 2aY. Morita, S. Suzuki, K. Sato, T. Takui, Nat. Chem. 2011, 3, 197–204;
- 2bZ. Sun, Q. Ye, C. Chi, J. Wu, Chem. Soc. Rev. 2012, 41, 7857–7889;
- 2cZ. Sun, Z. Zeng, J. Wu, Acc. Chem. Res. 2014, 47, 2582–2591;
- 2dH. Miyoshi, S. Nobusue, A. Shimizu, Y. Tobe, Chem. Soc. Rev. 2015, 44, 6560–6577;
- 2eT. Kubo, Chem. Rec. 2015, 15, 218–232.
- 3
- 3aZ. Zeng, X. L. Shi, C. Chi, J. T. López Navarrete, J. Casado, J. Wu, Chem. Soc. Rev. 2015, 44, 6578–6596;
- 3bJ. Casado, Top. Curr. Chem. 2017, 375, 73.
- 4
- 4aJ. Casado, R. Ponce Ortiz, J. T. López Navarrete, Chem. Soc. Rev. 2012, 41, 5672–5686;
- 4bY. Suzuki, E. Miyazaki, K. Takimiya, J. Am. Chem. Soc. 2010, 132, 10453–10466;
- 4cP. M. Burrezo, J. L. Zafra, J. T. L. Navarrete, J. Casado, Angew. Chem. Int. Ed. 2017, 56, 2250–2259; Angew. Chem. 2017, 129, 2286–2296.
- 5
- 5aT. Takahashi, K. Matsuoka, K. Takimiya, T. Otsubo, Y. Aso, J. Am. Chem. Soc. 2005, 127, 8928–8929;
- 5bR. Ponce Ortiz, J. Casado, V. Hernández, J. T. L. Navarrete, P. M. Viruela, E. Ortí, K. Takimiya, T. Otsubo, Angew. Chem. Int. Ed. 2007, 46, 9057–9061; Angew. Chem. 2007, 119, 9215–9219.
- 6
- 6aF. Liu, G. L. Espejo, S. Qiu, M. M. Oliva, J. Pina, J. S. Seixas de Melo, J. Casado, X. Zhu, J. Am. Chem. Soc. 2015, 137, 10357–10366;
- 6bC. Zhang, X. Zhu, Acc. Chem. Res. 2017, 50, 1342–1350.
- 7
- 7aA. Pron, P. Berrouard, M. Leclerc, Macromol. Chem. Phys. 2013, 214, 7–16;
- 7bP. He, X.-L. Qiao, Q. Qian, H.-X. Li, Chin. Chem. Lett. 2016, 27, 1277–1285.
- 8D. Yuan, D. Huang, S. Medina Rivero, A. Carreras, C. Zhang, Y. Zou, X. Jiao, C. R. McNeill, X. Zhu, C. Di, D. Zhu, D. Casanova, J. Casado, Chem 2019, 5, 964–976.
- 9P. Berrouard, F. Grenier, J.-R. Pouliot, E. Gagnon, C. Tessier, M. Leclerc, Org. Lett. 2011, 13, 38–41.
- 10C. Zhang, Y. Zang, E. Gann, C. R. McNeill, X. Zhu, C.-a. Di, D. Zhu, J. Am. Chem. Soc. 2014, 136, 16176–16184.
- 11See:
- 11aT. Kubo, M. Sakamoto, M. Akabane, Y. Fujiwara, K. Yamamoto, M. Akita, K. Inoue, T. Takui, K. Nakasuji, Angew. Chem. Int. Ed. 2004, 43, 6474–6479; Angew. Chem. 2004, 116, 6636–6641;
- 11bT. Kubo, A. Shimizu, M. Sakamoto, M. Uruichi, K. Yakushi, M. Nakano, D. Shiomi, K. Sato, T. Takui, Y. Morita, K. Nakasuji, Angew. Chem. Int. Ed. 2005, 44, 6564–6568; Angew. Chem. 2005, 117, 6722–6726;
- 11cA. Shimizu, M. Uruichi, K. Yakushi, H. Matsuzaki, H. Okamoto, M. Nakano, Y. Hirao, K. Matsumoto, H. Kurata, T. Kubo, Angew. Chem. Int. Ed. 2009, 48, 5482–5486; Angew. Chem. 2009, 121, 5590–5594;
- 11dA. Shimizu, R. Kishi, M. Nakano, D. Shiomi, K. Sato, T. Takui, I. Hisaki, M. Miyata, Y. Tobe, Angew. Chem. Int. Ed. 2013, 52, 6076–6079; Angew. Chem. 2013, 125, 6192–6195.
- 12B. Bleaney, K. D. Bowers, Proc. R. Soc. London Ser. A 1952, 214, 451.
- 13S. Canola, J. Casado, F. Negri, Phys. Chem. Chem. Phys. 2018, 20, 24227–24238.
- 14D. Yuan, S. Rivero Medina, L. Ren, M. E. Sandoval-Salinas, S. J. Grabowski, D. Casanova, X. Zhu, J. Casado, Chem. Eur. J. 2018, 24, 13523–13534.
- 15T. M. Pappenfus, J. D. Raff, E. J. Hukkanen, J. R. Burney, J. Casado, S. M. Drew, L. L. Miller, K. R. Mann, J. Org. Chem. 2002, 67, 6015–6024.
- 16
- 16aB. Müller, T. Bally, F. Gerson, A. de Meijere, M. von Seebach, J. Am. Chem. Soc. 2003, 125, 13776–13783;
- 16bT. Bally, Nat. Chem. 2010, 2, 165–166.
- 17A. Heckmann, C. Lambert, Angew. Chem. Int. Ed. 2012, 51, 326–392; Angew. Chem. 2012, 124, 334–404.
- 18J. Casado, L. L. Miller, K. R. Mann, T. M. Pappenfus, H. Higuchi, E. Orti, B. Milian, R. Pou-Amerigo, V. Hernandez, J. T. L. Navarrete, J. Am. Chem. Soc. 2002, 124, 12380–12388.