Chemoenzymatic Synthesis of DSGb5 and Sialylated Globo-series Glycans
Graphical Abstract
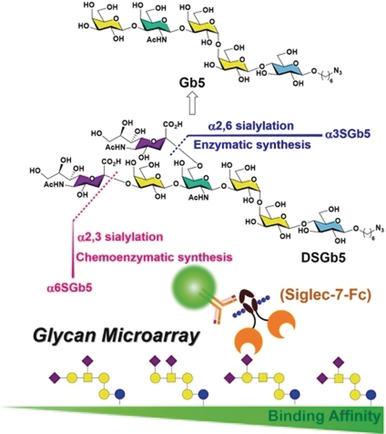
Sialyl-globo-series glycans, including DSGb5 and sialylglobosides, have been prepared using both a one-pot multiple enzyme synthesis method and chemoenzymatic synthesis. The resulting glycans were screened for binding to sialic-acid-binding, immunoglobulin-type lectin-7 (siglet-7) using a glycan-microarray-based binding assay.
Abstract
Sialic-acid-binding, immunoglobulin-type lectin-7 (Siglec-7) is present on the surface of natural killer cells. Siglec-7 shows preference for disialylated glycans, including α(2,8)-α(2,3)-disialic acids or internally branched α(2,6)-NeuAc, such as disialosylglobopentaose (DSGb5). Herein, DSGb5 was synthesized by a one-pot multiple enzyme method from Gb5 by α2,3-sialylation (with PmST1) followed by α2,6-sialylation (with Psp2,6ST) in 23 % overall yield. DSGb5 was also chemoenzymatically synthesized. The protection of the nonreducing-end galactose of Gb5 as 3,4-O-acetonide, 3,4-O-benzylidene, and 4,6-O-benzylidene derivatives provided DSGb5 in overall yields of 26 %, 12 %, and 19 %, respectively. Gb3, Gb4, and Gb5 were enzymatically sialylated to afford a range of globo-glycans. Surprisingly, DSGb5 shows a low affinity for Siglec-7 in a glycan microarray binding affinity assay. Among the synthesized globo-series glycans, α6α3DSGb4 shows the highest binding affinity for Siglec-7.