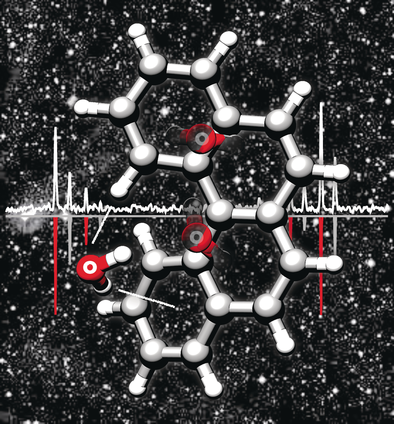
Water Docking Bias in [4]Helicene
Corresponding Author
Dr. Sérgio R. Domingos
Deutsches Elektronen-Synchrotron DESY, Notkestraße 85, 22607 Hamburg, Germany
Search for more papers by this authorDr. Kévin Martin
MOLTECH-Anjou, UMR 6200, CNRS, UNIV Angers, 2 bd Lavoisier, 49045 Angers Cedex, France
Search for more papers by this authorDr. Narcis Avarvari
MOLTECH-Anjou, UMR 6200, CNRS, UNIV Angers, 2 bd Lavoisier, 49045 Angers Cedex, France
Search for more papers by this authorCorresponding Author
Prof. Dr. Melanie Schnell
Deutsches Elektronen-Synchrotron DESY, Notkestraße 85, 22607 Hamburg, Germany
Institut für Physikalische Chemie, Christian-Albrechts-Universität zu Kiel, Max-Eyth-Straße 1, 24118 Kiel, Germany
Search for more papers by this authorCorresponding Author
Dr. Sérgio R. Domingos
Deutsches Elektronen-Synchrotron DESY, Notkestraße 85, 22607 Hamburg, Germany
Search for more papers by this authorDr. Kévin Martin
MOLTECH-Anjou, UMR 6200, CNRS, UNIV Angers, 2 bd Lavoisier, 49045 Angers Cedex, France
Search for more papers by this authorDr. Narcis Avarvari
MOLTECH-Anjou, UMR 6200, CNRS, UNIV Angers, 2 bd Lavoisier, 49045 Angers Cedex, France
Search for more papers by this authorCorresponding Author
Prof. Dr. Melanie Schnell
Deutsches Elektronen-Synchrotron DESY, Notkestraße 85, 22607 Hamburg, Germany
Institut für Physikalische Chemie, Christian-Albrechts-Universität zu Kiel, Max-Eyth-Straße 1, 24118 Kiel, Germany
Search for more papers by this authorGraphical Abstract
Abstract
We report on the one- and two-water clusters of [4]helicene, the smallest polycyclic aromatic hydrocarbon with a helical sense, which were captured in the gas phase using high-resolution rotational spectroscopy. The structures of the complexes are unambiguously revealed using microwave spectra of isotopically enriched species. In the one-water cluster, the apparent splitting pattern is consistent with a tunneling motion that encompasses an exchange of strongly and weakly bonded water hydrogens. This motion is “locked” in the two-water cluster. The relevant intermolecular contacts, symmetry, and aromaticity effects are unveiled for the microsolvated chiral topologies. These observations entail the first glance at the structures and internal dynamics of the water binding motifs of a chiral polycyclic aromatic hydrocarbon.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201902889-sup-0001-misc_information.pdf1.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1E. Peeters, L. Allamandola, D. Hudgins, S. Hony, A. Tielens, The Unidentified Infrared Features After ISO, ASP Conf. Ser. 309, Astrophysics of Dust, Eds.: A. N. Witt, G. C. Clayton, B. T. Draine, San Francisco, CA: ASP, 2004.
- 2A. Tielens, Annu. Rev. Astron. Astrophys. 2008, 46, 289–337.
- 3J. Bouwman, A. L. Mattioda, H. Linnartz, L. J. Allamandola, Astron. Astrophys. 2011, 525, A 93.
- 4A. M. Cook, A. Ricca, A. L. Mattioda, J. Bouwman, J. Roser, H. Linnartz, J. Bregman, L. J. Allamandola, Astrophys. J. 2015, 799, 14.
- 5A. L. Steber, C. Pérez, B. Temelso, G. C. Shields, A. M. Rijs, B. H. Pate, Z. Kisiel, M. Schnell, J. Phys. Chem. Lett. 2017, 8, 5744–5750.
- 6G. H. Wagniere, On Chirality and the Universal Asymmetry, Wiley-VCH, Weinheim, 2007.
- 7R. Gershoni-Poranne, A. P. Rahalkar, A. Stanger, Phys. Chem. Chem. Phys. 2018, 20, 14808–14817.
- 8F. L. Hirshfeld, S. Sandler, G. M. J. Schmidt, J. Chem. Soc. 1963, 2108–2125.
- 9G. G. Brown, B. C. Dian, K. O. Douglass, S. M. Geyer, S. T. Shipman, B. H. Pate, Rev. Sci. Instrum. 2008, 79, 053103.
- 10S. Blanco, P. Pinacho, J. C. López, Angew. Chem. Int. Ed. 2016, 55, 9331–9335; Angew. Chem. 2016, 128, 9477–9481.
- 11S. R. Domingos, C. Pérez, C. Medcraft, P. Pinacho, M. Schnell, Phys. Chem. Chem. Phys. 2016, 18, 16682–16689.
- 12C. Pérez, J. L. Neill, M. T. Muckle, D. P. Zaleski, I. Peña, J. C. Lopez, J. L. Alonso, B. H. Pate, Angew. Chem. Int. Ed. 2015, 54, 979–982; Angew. Chem. 2015, 127, 993–996.
- 13C. Pérez, A. Krin, A. L. Steber, J. C. López, Z. Kisiel, M. Schnell, J. Phys. Chem. Lett. 2016, 7, 154–160.
- 14S. R. Domingos, C. Pérez, M. Schnell, J. Chem. Phys. 2016, 145, 161103.
- 15E. G. Schnitzler, C. Badran, W. Jager, J. Phys. Chem. Lett. 2016, 7, 1143–1147.
- 16S. R. Domingos, A. Cnossen, W. J. Buma, W. R. Browne, B. L. Feringa, M. Schnell, Angew. Chem. Int. Ed. 2017, 56, 11209–11212; Angew. Chem. 2017, 129, 11361–11364.
- 17I. Uriarte, S. Melandri, A. Maris, C. Calabrese, E. J. Cocinero, J. Phys. Chem. Lett. 2018, 9, 1497–1502.
- 18E. R. Alonso, I. Leon, L. Kolesnikova, J. L. Alonso, ChemPhysChem 2018, 19, 3334–3340.
- 19D. Loru, A. L. Steber, C. Pérez, S. Gruet, M. Schnell, International Symposium on Molecular Spectroscopy, 2018.
- 20M. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. Hratchian, A. Izmaylov, J. Bloino, G. Zheng, J. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. Montgomery, J. Peralta, F. Ogliaro, M. Bearpark, J. Heyd, E. Brothers, K. Kudin, V. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. Burant, S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. Millam, M. Klene, J. Knox, J. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. Stratmann, O. Yazyev, A. Austin, R. Cammi, C. Pomelli, J. Ochterski, R. Martin, K. Morokuma,V. Zakrzewski, G.Voth, P. Salvador, J. Dannenberg, S. Dapprich, A. Daniels, O. Farkas, J. Foresman, J. Ortiz, J. Cioslowski, D. Fox, Gaussian 09, revision C.02; Gaussian, Inc.: Wallingford, CT, 2009.
- 21D. Schmitz, V. Alvin Shubert, T. Betz, M. Schnell, J. Mol. Spectrosc. 2012, 280, 77–84.
- 22C. Pérez, S. Lobsiger, N. A. Seifert, D. P. Zaleski, B. Temelso, G. C. Shields, Z. Kisiel, B. H. Pate, Chem. Phys. Lett. 2013, 571, 1–15.
- 23K. Suma, Y. Sumiyoshi, Y. Endo, Science 2006, 311, 1278–1281.
- 24L. Evangelisti, W. Caminati, Phys. Chem. Chem. Phys. 2010, 12, 14433–14441.
- 25J. O. Richardson, C. Pérez, S. Lobsiger, A. A. Reid, B. Temelso, G. C. Shields, Z. Kisiel, D. J. Wales, B. H. Pate, S. C. Althorpe, Science 2016, 351, 1310–1313.
- 26W. Huang, J. Thomas, W. Jager, Y. Xu, Phys. Chem. Chem. Phys. 2017, 19, 12221–12228.
- 27C. Western, J. Quant. Spectrosc. Radiat. Transfer 2017, 186, 221–242.
- 28S. Grimme, J. Antony, S. Ehrlich, H. Krieg, J. Chem. Phys. 2010, 132, 154104.
- 29J. Kraitchman, Am. J. Phys. 1953, 21, 17–24.
- 30Z. Kisiel, J. Mol. Spectrosc. 2003, 218, 58–67.
- 31K. Kisiel, PROSPE: Programs for Rotational Spectroscopy.
- 32J. Contreras-García, E. R. Johnson, S. Keinan, R. Chaudret, J.-P. Piquemal, D. N. Beratan, W. Yang, J. Chem. Theory Comput. 2011, 7, 625–632.
- 33L. Evangelisti, K. Brendel, H. Mäder, W. Caminati, S. Melandri, Angew. Chem. Int. Ed. 2017, 56, 13699–13703; Angew. Chem. 2017, 129, 13887–13891.
- 34C. Pérez, I. León, A. Lesarri, B. H. Pate, R. Martínez, J. Millán, J. A. Fernández, Angew. Chem. Int. Ed. 2018, 57, 15112–15116; Angew. Chem. 2018, 130, 15332–15336.
- 35B. Jeziorski, R. Moszynski, K. Szalewicz, Chem. Rev. 1994, 94, 1887–1930.
- 36C. Pérez, A. L. Steber, A. M. Rijs, B. Temelso, G. C. Shields, J. C. Lopez, Z. Kisiel, M. Schnell, Phys. Chem. Chem. Phys. 2017, 19, 14214–14223.
- 37J. Kruszewski, T. Krygowski, Tetrahedron Lett. 1972, 13, 3839–3842.
- 38Z. Chen, C. S. Wannere, C. Corminboeuf, R. Puchta, P. v. R. Schleyer, Chem. Rev. 2005, 105, 3842–3888.