Significant Polar Comonomer Enchainment in Zirconium-Catalyzed, Masking Reagent-Free, Ethylene Copolymerizations
Jiazhen Chen
Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL, 60208 USA
Search for more papers by this authorDr. Alessandro Motta
Dipartimento di Scienze Chimiche, Università di Roma “La Sapienza” and INSTM, UdR Roma, Piazzale Aldo Moro 5, 00185 Roma, Italy
Search for more papers by this authorDr. Binghao Wang
Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL, 60208 USA
Search for more papers by this authorCorresponding Author
Dr. Yanshan Gao
Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL, 60208 USA
Search for more papers by this authorCorresponding Author
Prof. Tobin J. Marks
Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL, 60208 USA
Search for more papers by this authorJiazhen Chen
Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL, 60208 USA
Search for more papers by this authorDr. Alessandro Motta
Dipartimento di Scienze Chimiche, Università di Roma “La Sapienza” and INSTM, UdR Roma, Piazzale Aldo Moro 5, 00185 Roma, Italy
Search for more papers by this authorDr. Binghao Wang
Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL, 60208 USA
Search for more papers by this authorCorresponding Author
Dr. Yanshan Gao
Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL, 60208 USA
Search for more papers by this authorCorresponding Author
Prof. Tobin J. Marks
Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL, 60208 USA
Search for more papers by this authorGraphical Abstract
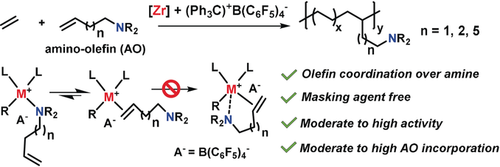
Masking reagent-free: Organozirconium-catalyzed copolymerization of ethylene with polar amino-olefins proceeds with high activity and high polar monomer incorporation in the absence of a masking reagent. Experimental and DFT mechanistic analysis show that the high activity is attributable to favorable olefin over amino group coordination.
Abstract
In principal, the direct copolymerization of ethylene with polar comonomers should be the most efficient means to introduce functional groups into conventional polyolefins but remains a formidable challenge. Despite the tremendous advances in group 4-centered catalysis for olefin polymerization, successful examples of ethylene + polar monomer copolymerization are rare, especially without Lewis acidic masking reagents. Here we report that certain group 4 catalysts are very effective for ethylene + CH2=CH(CH2)nNR2 copolymerizations with activities up to 3400 Kg copolymer mol−1-Zr h-1 atm-1, and with comonomer enchainment up to 5.5 mol % in the absence of masking reagents. Group 4 catalyst-amino-olefin structure–activity-selectivity relationships reflect the preference of olefin activation over free amine coordination, which is supported by mechanistic experiments and DFT analysis. These results illuminate poorly understood facets of d0 metal-catalyzed polar olefin monomer copolymerization processes.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201902042-sup-0001-misc_information.pdf1.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. B. Williamson, W. L. Czaplyski, E. J. Alexanian, F. A. Leibfarth, Angew. Chem. Int. Ed. 2018, 57, 6261–6265; Angew. Chem. 2018, 130, 6369–6373;
- 1bC. J. Kay, P. D. Goring, C. A. Burnett, B. Hornby, K. Lewtas, S. Morris, C. Morton, T. McNally, G. W. Theaker, C. Waterson, P. M. Wright, P. Scott, J. Am. Chem. Soc. 2018, 140, 13921–13934;
- 1cA. Bunescu, S. Lee, Q. Li, J. F. Hartwig, ACS Cent. Sci. 2017, 3, 895–903;
- 1dM. Stürzel, S. Mihan, R. Mülhaupt, Chem. Rev. 2016, 116, 1398–1433;
- 1eA. Kermagoret, A. Debuigne, C. Jerome, C. Detrembleur, Nat. Chem. 2014, 6, 179–187;
- 1fT. C. M. Chung, Macromolecules 2013, 46, 6671–6698;
- 1gN. M. G. Franssen, J. N. H. Reek, B. de Bruin, Chem. Soc. Rev. 2013, 42, 5809–5832.
- 2C. Chen, Nat. Rev. Chem. 2018, 2, 6–14.
- 3
- 3aH. Wang, Y. Yang, M. Nishiura, Y. Higaki, A. Takahara, Z. Hou, J. Am. Chem. Soc. 2019, 141, 3249–3257;
- 3bD. Liu, M. Wang, Y. Chai, X. Wan, D. Cui, ACS Catal. 2019, 9, 2618–2625;
- 3cB. Liu, K. Qiao, J. Fang, T. Wang, Z. Wang, D. Liu, Z. Xie, L. Maron, D. Cui, Angew. Chem. Int. Ed. 2018, 57, 14896–14901; Angew. Chem. 2018, 130, 15112–15117;
- 3dS. Li, D. Liu, Z. Wang, D. Cui, ACS Catal. 2018, 8, 6086–6093;
- 3eJ. Chen, Y. Gao, B. Wang, T. L. Lohr, T. J. Marks, Angew. Chem. Int. Ed. 2017, 56, 15964–15968; Angew. Chem. 2017, 129, 16180–16184;
- 3fD. Liu, M. Wang, Z. Wang, C. Wu, Y. Pan, D. Cui, Angew. Chem. Int. Ed. 2017, 56, 2714–2719; Angew. Chem. 2017, 129, 2758–2763;
- 3gC. Wang, G. Luo, M. Nishiura, G. Song, A. Yamamoto, Z. Hou, Y. Luo, Z. Hou, Sci. Adv. 2017, 3, e 1701011;
- 3hD. Liu, C. Yao, R. Wang, M. Wang, Z. Wang, C. Wu, F. Lin, S. Li, X. Wan, D. Cui, Angew. Chem. Int. Ed. 2015, 54, 5205–5209; Angew. Chem. 2015, 127, 5294–5298.
- 4
- 4aL. S. Boffa, B. M. Novak, Chem. Rev. 2000, 100, 1479–1493;
- 4bU. M. Stehling, K. M. Stein, D. Fischer, R. M. Waymouth, Macromolecules 1999, 32, 14–20;
- 4cU. M. Stehling, K. M. Stein, M. R. Kesti, R. M. Waymouth, Macromolecules 1998, 31, 2019–2027;
- 4dM. R. Kesti, G. W. Coates, R. M. Waymouth, J. Am. Chem. Soc. 1992, 114, 9679–9680.
- 5
- 5aX.-H. Yang, C.-R. Liu, C. Wang, X.-L. Sun, Y.-H. Guo, X.-K. Wang, Z. Wang, Z. Xie, Y. Tang, Angew. Chem. Int. Ed. 2009, 48, 8099–8102; Angew. Chem. 2009, 121, 8243–8246;
- 5bH. Terao, S. Ishii, M. Mitani, H. Tanaka, T. Fujita, J. Am. Chem. Soc. 2008, 130, 17636–17637.
- 6
- 6aC. Tan, C. Chen, Angew. Chem. Int. Ed. 2019, https://doi.org/10.1002/anie.201814634; Angew. Chem. 2019, https://doi.org/10.1002/ange.201814634;
- 6bH. Mu, L. Pan, D. Song, Y. Li, Chem. Rev. 2015, 115, 12091–12137;
- 6cB. P. Carrow, K. Nozaki, Macromolecules 2014, 47, 2541–2555.
- 7
- 7aY. Ota, S. Ito, M. Kobayashi, S. Kitade, K. Sakata, T. Tayano, K. Nozaki, Angew. Chem. Int. Ed. 2016, 55, 7505–7509; Angew. Chem. 2016, 128, 7631–7635;
- 7bZ. Chen, W. Liu, O. Daugulis, M. Brookhart, J. Am. Chem. Soc. 2016, 138, 16120–16129;
- 7cS. Dai, C. Chen, Angew. Chem. Int. Ed. 2016, 55, 13281–13285; Angew. Chem. 2016, 128, 13475–13479;
- 7dB. K. Long, J. M. Eagan, M. Mulzer, G. W. Coates, Angew. Chem. Int. Ed. 2016, 55, 7106–7110; Angew. Chem. 2016, 128, 7222–7226.
- 8
- 8aY. Gao, J. Chen, Y. Wang, D. B. Pickens, A. Motta, Q. J. Wang, Y.-W. Chung, T. L. Lohr, T. J. Marks, Nat. Catal. 2019, 2, 236–242;
- 8bJ. P. McInnis, M. Delferro, T. J. Marks, Acc. Chem. Res. 2014, 47, 2545–2557;
- 8cH. Makio, H. Terao, A. Iwashita, T. Fujita, Chem. Rev. 2011, 111, 2363–2449.
- 9
- 9aX. Wang, Y. Wang, X. Shi, J. Liu, C. Chen, Y. Li, Macromolecules 2014, 47, 552–559;
- 9bZ. Chen, J.-F. Li, W.-J. Tao, X.-L. Sun, X.-H. Yang, Y. Tang, Macromolecules 2013, 46, 2870–2875.
- 10
- 10aL. Zhang, M. Nishiura, M. Yuki, Y. Luo, Z. Hou, Angew. Chem. Int. Ed. 2008, 47, 2642–2645; Angew. Chem. 2008, 120, 2682–2685;
- 10bE. Y.-X. Chen, T. J. Marks, Chem. Rev. 2000, 100, 1391–1434.
- 11Y. Na, D. Zhang, C. Chen, Polym. Chem. 2017, 8, 2405–2409.
- 12
- 12aZ. Shi, F. Guo, Y. Li, Z. Hou, J. Polym. Sci. Part A 2015, 53, 5–9;
- 12bJ. Xu, G. Chen, R. Yan, D. Wang, M. Zhang, W. Zhang, P. Sun, Macromolecules 2011, 44, 3730–3738.
- 13M. Zhang, X. Yuan, L. Wang, T. C. M. Chung, T. Huang, W. de Groot, Macromolecules 2014, 47, 571–581.
- 14Y.-X. Chen, M. V. Metz, L. Li, C. L. Stern, T. J. Marks, J. Am. Chem. Soc. 1998, 120, 6287–6305.
- 15F. Zaccaria, C. Ehm, P. H. M. Budzelaar, V. Busico, ACS Catal. 2017, 7, 1512–1519.
- 16M. R. Radlauer, A. K. Buckley, L. M. Henling, T. Agapie, J. Am. Chem. Soc. 2013, 135, 3784–3787.
- 17L. Resconi, F. Piemontesi, I. Camurati, O. Sudmeijer, I. E. Nifant'ev, P. V. Ivchenko, L. G. Kuz'mina, J. Am. Chem. Soc. 1998, 120, 2308–2321.
- 18X.-Y. Wang, Y.-Y. Long, Y.-X. Wang, Y.-S. Li, J. Polym. Sci. Part A 2014, 52, 3421–3428.
- 19T. R. Younkin, E. F. Connor, J. I. Henderson, S. K. Friedrich, R. H. Grubbs, D. A. Bansleben, Science 2000, 287, 460–462.