All-Fullerene Electron Donor–Acceptor Conjugates
Dr. Marta Izquierdo
Departamento de Química Orgánica, Facultad de Ciencias Químicas, Universidad Complutense, 28040 Madrid, Spain
These authors contributed equally to this work.
Search for more papers by this authorBenedikt Platzer
Interdisciplinary Center for Molecular Materials, Department of Chemistry and Pharmacy, Friedrich-Alexander Universität Erlangen-Nürnberg, Egerlandstr. 3, 91058 Erlangen, Germany
These authors contributed equally to this work.
Search for more papers by this authorDr. Anton J. Stasyuk
Institute of Computational Chemistry and Catalysis and Department of Chemistry, University of Girona, C/M. Aurèlia Capmany, 69, 17003 Girona, Spain
Search for more papers by this authorDr. Olga A. Stasyuk
Institute of Computational Chemistry and Catalysis and Department of Chemistry, University of Girona, C/M. Aurèlia Capmany, 69, 17003 Girona, Spain
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of Czech Republic, Flemingo nám. 2, 16610 Prague, Czech Republic
Search for more papers by this authorProf. Dr. Alexander A. Voityuk
Institute of Computational Chemistry and Catalysis and Department of Chemistry, University of Girona, C/M. Aurèlia Capmany, 69, 17003 Girona, Spain
Institució Catalana de Recerca i Estudis Avancats (ICREA), 08010 Barcelona, Spain
Search for more papers by this authorSergio Cuesta
Departamento de Química Orgánica, Facultad de Ciencias Químicas, Universidad Complutense, 28040 Madrid, Spain
Search for more papers by this authorCorresponding Author
Prof. Dr. Miquel Solà
Institute of Computational Chemistry and Catalysis and Department of Chemistry, University of Girona, C/M. Aurèlia Capmany, 69, 17003 Girona, Spain
Search for more papers by this authorCorresponding Author
Prof. Dr. Dirk M. Guldi
Interdisciplinary Center for Molecular Materials, Department of Chemistry and Pharmacy, Friedrich-Alexander Universität Erlangen-Nürnberg, Egerlandstr. 3, 91058 Erlangen, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Nazario Martín
Departamento de Química Orgánica, Facultad de Ciencias Químicas, Universidad Complutense, 28040 Madrid, Spain
Search for more papers by this authorDr. Marta Izquierdo
Departamento de Química Orgánica, Facultad de Ciencias Químicas, Universidad Complutense, 28040 Madrid, Spain
These authors contributed equally to this work.
Search for more papers by this authorBenedikt Platzer
Interdisciplinary Center for Molecular Materials, Department of Chemistry and Pharmacy, Friedrich-Alexander Universität Erlangen-Nürnberg, Egerlandstr. 3, 91058 Erlangen, Germany
These authors contributed equally to this work.
Search for more papers by this authorDr. Anton J. Stasyuk
Institute of Computational Chemistry and Catalysis and Department of Chemistry, University of Girona, C/M. Aurèlia Capmany, 69, 17003 Girona, Spain
Search for more papers by this authorDr. Olga A. Stasyuk
Institute of Computational Chemistry and Catalysis and Department of Chemistry, University of Girona, C/M. Aurèlia Capmany, 69, 17003 Girona, Spain
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of Czech Republic, Flemingo nám. 2, 16610 Prague, Czech Republic
Search for more papers by this authorProf. Dr. Alexander A. Voityuk
Institute of Computational Chemistry and Catalysis and Department of Chemistry, University of Girona, C/M. Aurèlia Capmany, 69, 17003 Girona, Spain
Institució Catalana de Recerca i Estudis Avancats (ICREA), 08010 Barcelona, Spain
Search for more papers by this authorSergio Cuesta
Departamento de Química Orgánica, Facultad de Ciencias Químicas, Universidad Complutense, 28040 Madrid, Spain
Search for more papers by this authorCorresponding Author
Prof. Dr. Miquel Solà
Institute of Computational Chemistry and Catalysis and Department of Chemistry, University of Girona, C/M. Aurèlia Capmany, 69, 17003 Girona, Spain
Search for more papers by this authorCorresponding Author
Prof. Dr. Dirk M. Guldi
Interdisciplinary Center for Molecular Materials, Department of Chemistry and Pharmacy, Friedrich-Alexander Universität Erlangen-Nürnberg, Egerlandstr. 3, 91058 Erlangen, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Nazario Martín
Departamento de Química Orgánica, Facultad de Ciencias Químicas, Universidad Complutense, 28040 Madrid, Spain
Search for more papers by this authorDedicated to Professor Jean-Marie Lehn on the occasion of his 80th birthday
Graphical Abstract
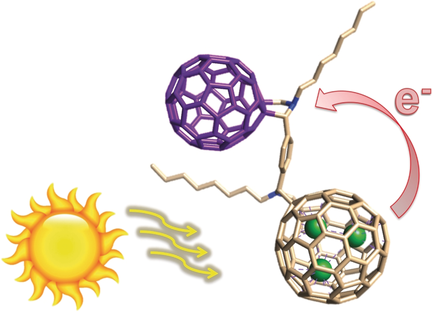
Dumbbell charge transfer: The synthesis of an all-fullerene electron donor–acceptor conjugate, C60-Lu3N@Ih-C80, is reported. In the dumbbell-like system, one fullerene moiety acts as the donor (Lu3N@Ih-C80) while the other acts as the acceptor (C60). Spin conversion of the charge-separated singlet state into the corresponding triplet state slows down the charge recombination by one order of magnitude.
Abstract
The synthesis and characterization of a covalent all-fullerene C60-Lu3N@Ih-C80 electron donor–acceptor conjugate has been realized by sequential 1,3-dipolar cycloaddition reactions of azomethine ylides on Lu3N@Ih-C80 and C60. To the best of our knowledge, this is the first time that two fullerenes behaving as both electron donor (Lu3N@Ih-C80) and acceptor (C60) are forming an electroactive dumbbell. DFT calculations reveal up to 16 diastereomeric pairs, that is, 8 with syn and 8 with anti orientation, with the anti-RSSS isomer being the most stable. Spectroelectrochemical absorption and femtosecond transient absorption experiments support the notion that a C60⋅−-Lu3N@Ih-C80⋅+ charge-separated state is formed. Spin conversion from the charge-separated singlet state C60⋅−-Lu3N@Ih-C80⋅+ into the corresponding triplet state is facilitated by the heavy-atom effect stemming from the Lu3N-cluster, which, in turn, slows down the charge recombination by one order of magnitude.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201901863-sup-0001-misc_information.pdf2.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. R. Wasielewski, Acc. Chem. Res. 2009, 42, 1910–1921.
- 2 Energy Harvesting Materials (Ed.: ), World Scientific Publishing Co., Singapore, 2005.
- 3
- 3aH. Imahori, D. M. Guldi, K. Tamaki, Y. Yoshida, C. Luo, Y. Sakata, S. Fukuzumi, J. Am. Chem. Soc. 2001, 123, 6617–6628;
- 3bS. Fukuzumi, K. Ohkubo, T. Suenobu, Acc. Chem. Res. 2014, 47, 1455–1464;
- 3cC. B. KC, G. N. Lim, F. D'Souza, Angew. Chem. Int. Ed. 2015, 54, 5088–5092; Angew. Chem. 2015, 127, 5177–5181;
- 3dL. Moreira, J. Calbo, J. Aragó, B. M. Illescas, I. Nierengarten, B. Delavaux-Nicot, E. Ortí, N. Martín, J.-F. Nierengarten, J. Am. Chem. Soc. 2016, 138, 15359–15367.
- 4
- 4aN. Martín, L. Sánchez, B. Illescas, I. Pérez, Chem. Rev. 1998, 98, 2527–2548;
- 4bA. Zieleniewska, F. Lodermeyer, A. Roth, D. M. Guldi, Chem. Soc. Rev. 2018, 47, 702–714.
- 5
- 5aJ. R. Pinzón, C. M. Cardona, M. Á. Herranz, M. E. Plonska-Brzezinska, A. Palkar, A. J. Athans, N. Martín, A. Rodríguez-Fortea, J. M. Poblet, G. Bottari, T. Torres, S. S. Gayathri, D. M. Guldi, L. Echegoyen, Chem. Eur. J. 2009, 15, 864–877;
- 5bS. Wolfrum, J. R. Pinzón, A. Molina-Ontoria, A. Gouloumis, N. Martín, L. Echegoyen, D. M. Guldi, Chem. Commun. 2011, 47, 2270–2272;
- 5cA. Molina-Ontoria, M. Wielopolski, J. Gebhardt, A. Gouloumis, T. Clark, D. M. Guldi, N. Martín, J. Am. Chem. Soc. 2011, 133, 2370–2373;
- 5dH. Nikawa, Y. Araki, Z. Slanina, T. Tsuchiya, T. Akasaka, T. Wada, O. Ito, K.-P. Dinse, M. Ata, T. Kato, S. Nagase, Chem. Commun. 2010, 46, 631–633.
- 6Y. Takano, S. Obuchi, N. Mizorogi, R. García, M. Á. Herranz, M. Rudolf, D. M. Guldi, N. Martín, S. Nagase, T. Akasaka, J. Am. Chem. Soc. 2012, 134, 19401–19408.
- 7L. Feng, M. Rudolf, S. Wolfrum, A. Troeger, Z. Slanina, T. Akasaka, S. Nagase, N. Martín, T. Ameri, C. J. Brabec, D. M. Guldi, J. Am. Chem. Soc. 2012, 134, 12190–12197.
- 8
- 8aK. Sato, M. Kako, M. Suzuki, N. Mizorogi, T. Tsuchiya, M. M. Olmstead, A. L. Balch, T. Akasaka, S. Nagase, J. Am. Chem. Soc. 2012, 134, 16033–16039;
- 8bN. Chen, J. R. Pinzón, L. Echegoyen, ChemPhysChem 2011, 12, 1422–1425;
- 8cM. Rudolf, L. Feng, Z. Slanina, W. Wang, S. Nagase, T. Akasaka, D. M. Guldi, Nanoscale 2016, 8, 13257–13262;
- 8dM. Rudolf, S. Wolfrum, D. M. Guldi, L. Feng, T. Tsuchiya, T. Akasaka, L. Echegoyen, Chem. Eur. J. 2012, 18, 5136–5148.
- 9D. M. Guldi, Chem. Commun. 2000, 321–327.
- 10
- 10aD. M. Guldi, N. Martín, J. Mater. Chem. 2002, 12, 1978–1992;
- 10bC. Atienza, N. Martín, M. Wielopolski, N. Haworth, T. Clark, D. M. Guldi, Chem. Commun. 2006, 3202–3204.
- 11M. R. Cerón, V. Maffeis, S. Stevenson, L. Echegoyen, Inorg. Chim. Acta 2017, 468, 16–27 and references therein; Compound 5: MALDI-TOF: MS(+) (m/z): [M]+ calculated for C90H21N2Lu3: 1655.043, found: 1655.022.
- 12
- 12aD. Zhou, H. Tan, C. Luo, L. Gan, C. Huang, J. Pan, M. Lü, Y. Wu, Tetrahedron Lett. 1995, 36, 9169–9172;
- 12bL. Gan, D. Zhou, C. Luo, H. Tan, C. Huang, M. Lü, J. Pan, Y. Wu, J. Org. Chem. 1996, 61, 1954–1961;
- 12cX. Lu, X. He, L. Feng, Z. Shi, Z. Gu, Tetrahedron 2004, 60, 3713–3716.
- 13M. N. Chaur, F. Melin, A. L. Ortiz, L. Echegoyen, Angew. Chem. Int. Ed. 2009, 48, 7514–7538; Angew. Chem. 2009, 121, 7650–7675.
- 14By means of this approach we have identified that Lu3N@Ih-C80/C60 interactions in syn-RRSS and syn-RSSS, phenyl/C60 interactions, and C60/aliphatic C8 chain interactions in anti-RSSS impact the relative stabilities. These interactions and the characteristics of selected BCPs are shown in Figure S15 and Table S1 in the Supporting Information.
- 15R. A. Marcus, N. Sutin, Biochim. Biophys. Acta Rev. Bioenerg. 1985, 811, 265–322.
- 16All experiments were performed with both isomers, 6 a and 6 b, without observing any notable differences. Thus, in the following, we focus our discussions on 6 a.
- 17M. Maggini, G. Scorrano, M. Prato, J. Am. Chem. Soc. 1993, 115, 9798–9799.
- 18B. Liu, H. Fang, X. Li, W. Cai, L. Bao, M. Rudolf, F. Plass, L. Fan, X. Lu, D. M. Guldi, Chem. Eur. J. 2015, 21, 746–752.
- 19No evidence for the involvement of any C60 excited triplet state in the overall deactivation cascade was noted (see: D. M. Guldi, M. Prato, Acc. Chem. Res. 2000, 33, 695–703).