Direct Catalytic Asymmetric Aldol Reaction of α-Alkoxyamides to α-Fluorinated Ketones
Dr. Roman Pluta
Institute of Microbial Chemistry (BIKAKEN), 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo, 141-0021 Japan
Search for more papers by this authorCorresponding Author
Dr. Naoya Kumagai
Institute of Microbial Chemistry (BIKAKEN), 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo, 141-0021 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Masakatsu Shibasaki
Institute of Microbial Chemistry (BIKAKEN), 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo, 141-0021 Japan
Search for more papers by this authorDr. Roman Pluta
Institute of Microbial Chemistry (BIKAKEN), 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo, 141-0021 Japan
Search for more papers by this authorCorresponding Author
Dr. Naoya Kumagai
Institute of Microbial Chemistry (BIKAKEN), 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo, 141-0021 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Masakatsu Shibasaki
Institute of Microbial Chemistry (BIKAKEN), 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo, 141-0021 Japan
Search for more papers by this authorGraphical Abstract
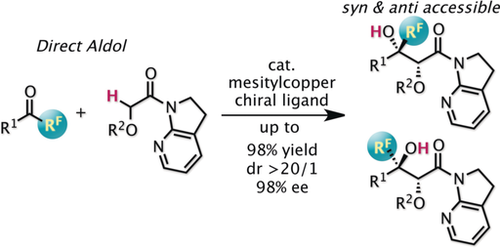
Combining oxygen and fluorine: α-Oxygen-functionalized amides were employed as enolate surrogates in a direct catalytic aldol coupling with α-fluorinated ketones. Because of the likely involvement of open transition states, both syn- and anti-aldol adducts can be accessed with high enantioselectivity by judicious choice of the chiral ligands.
Abstract
α-Oxygen-functionalized amides found particular utility as enolate surrogates for direct aldol couplings with α-fluorinated ketones in a catalytic manner. Because of the likely involvement of open transition states, both syn- and anti-aldol adducts can be accessed with high enantioselectivity by judicious choice of the chiral ligands. A broad variety of alkoxy substituents on the amides and aryl and fluoroalkyl groups on the ketone were tolerated, and the corresponding substrates delivered a range of enantioenriched fluorinated 1,2-dihydroxycarboxylic acid derivatives with divergent diastereoselectivity depending on the ligand used. The amide moiety of the aldol adduct was transformed into a variety of functional groups without protection of the tertiary alcohol, showcasing the synthetic utility of the present asymmetric aldol process.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201814607-sup-0001-misc_information.pdf14.7 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aK. Müller, C. Faeh, F. Diederich, Science 2007, 317, 1881;
- 1bS. Purser, P. R. Moore, S. Swallow, V. Gouverneur, Chem. Soc. Rev. 2008, 37, 320;
- 1cJ. Wang, M. Sanchez-Rosello, J. L. Acena, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok, H. Liu, Chem. Rev. 2014, 114, 2432;
- 1dE. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly, N. A. Meanwell, J. Med. Chem. 2015, 58, 8315;
- 1eY. Zhou, J. Wang, Z. Gu, S. Wang, W. Zhu, J. L. Acena, V. A. Soloshonok, K. Izawa, H. Liu, Chem. Rev. 2016, 116, 422;
- 1fN. A. Meanwell, J. Med. Chem. 2018, 61, 5822;
- 1gY. Zhu, J. Han, J. Wang, N. Shibata, M. Sodeoka, V. A. Soloshonok, J. A. S. Coelho, F. D. Toste, Chem. Rev. 2018, 118, 3887.
- 2
- 2aD. O'Hagan, Chem. Soc. Rev. 2008, 37, 308;
- 2bL. Hunter, Beilstein J. Org. Chem. 2010, 6, 38;
- 2cD. Cahard, V. Bizet, Chem. Soc. Rev. 2014, 43, 135;
- 2dM. G. Campbell, T. Ritter, Chem. Rev. 2015, 115, 612;
- 2eX. Yang, T. Wu, R. J. Phipps, F. D. Toste, Chem. Rev. 2015, 115, 826.
- 3
- 3aS. Mukherjee, J. W. Yang, S. Hoffmann, B. List, Chem. Rev. 2007, 107, 5471;
- 3bB. M. Trost, C. S. Brindle, Chem. Soc. Rev. 2010, 39, 1600;
- 3cR. Mahrwald, Modern Methods in Stereoselective Aldol Recations, Wiley-VCH, Weinheim, 2013, p. 1 online resource;
10.1002/9783527656714.ch1 Google Scholar
- 3dY. Yamashita, T. Yasukawa, W. J. Yoo, T. Kitanosono, S. Kobayashi, Chem. Soc. Rev. 2018, 47, 4388.
- 4
- 4aY. M. A. Yamada, N. Yoshikawa, H. Sasai, M. Shibasaki, Angew. Chem. Int. Ed. Engl. 1997, 36, 1871; Angew. Chem. 1997, 109, 1942;
- 4bN. Yoshikawa, Y. M. A. Yamada, J. Das, H. Sasai, M. Shibasaki, J. Am. Chem. Soc. 1999, 121, 4168;
- 4cB. List, R. A. Lerner, C. F. Barbas III, J. Am. Chem. Soc. 2000, 122, 2395;
- 4dB. M. Trost, H. Ito, J. Am. Chem. Soc. 2000, 122, 12003.
- 5W. Guoa, J. Weia, Y. Liua, C. Li, Tetrahedron 2014, 70, 6561.
- 6
- 6aL.-H. Qiu, Z.-X. Shen, C.-Q. Shi, Y.-H. Liu, Y.-W. Zhang, Chin. J. Chem. 2005, 23, 584;
- 6bX.-J. Wang, Y. Zhao, J.-T. Liu, Org. Lett. 2007, 9, 1343;
- 6cD. Zhang, C. Yuan, Tetrahedron 2008, 64, 2480;
- 6dN. Hara, R. Tamura, Y. Funahashi, S. Nakamura, Org. Lett. 2011, 13, 1662;
- 6eN. Duangdee, W. Harnying, G. Rulli, J. M. Neudörfl, H. Gröger, A. Berkessel, J. Am. Chem. Soc. 2012, 134, 11196;
- 6fC. G. Kokotos, J. Org. Chem. 2012, 77, 1131;
- 6gY. H. Deng, J. Q. Chen, L. He, T. R. Kang, Q. Z. Liu, S. W. Luo, W. C. Yuan, Chem. Eur. J. 2013, 19, 7143;
- 6hJ. Lin, T. Kang, Q. Liu, L. He, Tetrahedron: Asymmetry 2014, 25, 949;
- 6iW. Yang, Y.-M. Cui, W. Zhou, L. Li, K.-F. Yang, Z.-J. Zheng, Y. Lu, L.-W. Xu, Synlett 2014, 25, 1461;
- 6jH. Zong, H. Huang, G. Bian, L. Song, J. Org. Chem. 2014, 79, 11768;
- 6kL. M. Lutete, T. Miyamoto, T. Ikemoto, Tetrahedron Lett. 2016, 57, 1220;
- 6lP. Wang, H. F. Li, J. Z. Zhao, Z. H. Du, C. S. Da, Org. Lett. 2017, 19, 2634.
- 7For catalytic asymmetric aldol reactions of trifluoromethyl ketones using highly acidic aldol donors, see: diazoacetate:
- 7aH.-F. Cui, L. Wang, L.-J. Yang, J. Nie, Y. Zheng, J.-A. Ma, Tetrahedron 2011, 67, 8470; 1,3-dicarbonyl compounds:
- 7bX.-J. Li, H.-Y. Xiong, M.-Q. Hua, J. Nie, Y. Zheng, J.-A. Ma, Tetrahedron 2012, 53, 2117;
- 7cY. Zheng, H. Y. Xiong, J. Nie, M. Q. Hua, J. A. Ma, Chem. Commun. 2012, 48, 4308.
- 8For NHC-promoted oxidative couplings of aldehydes and trifluoromethyl ketones, see: J. Mo, R. Yang, X. Chen, B. Tiwari, Y. R. Chi, Org. Lett. 2013, 15, 50.
- 9For an intriguing example using β,γ-unsaturated ketones for a vinylogous aldol reaction at the γ-position, see: Z. Jing, X. Bai, W. Chen, G. Zhang, B. Zhu, Z. Jiang, Org. Lett. 2016, 18, 260.
- 10
- 10aS. Saito, S. Kobayashi, J. Am. Chem. Soc. 2006, 128, 8704;
- 10bK. Ishizawa, H. Nagai, Y. Shimizu, M. Kanai, Chem. Pharm. Bull. 2018, 66, 231.
- 11For comprehensive reviews on the use of C1-ammonium enolates in Lewis basic organocatalysis to give ester and amide products, see:
- 11aM. J. Gaunt, C. C. C. Johansson, Chem. Rev. 2007, 107, 5596;
- 11bL. C. Morrill, A. D. Smith, Chem. Soc. Rev. 2014, 43, 6214.
- 12For selected leading examples of the use of C1-ammonium enolates in combination with Lewis acid co-catalysts, see:
- 12aC. J. Abraham, D. H. Paull, T. Bekele, M. T. Scerba, T. Dudding, T. Lectka, J. Am. Chem. Soc. 2008, 130, 17085;
- 12bJ. Erb, D. H. Paull, T. Dudding, L. Belding, T. Lectka, J. Am. Chem. Soc. 2011, 133, 7536.
- 13For a partially successful example of a direct catalytic asymmetric aldol reaction using α-fluorinated ynones as electrophiles, see: H. Noda, F. Amemiya, K. Weidner, N. Kumagai, M. Shibasaki, Chem. Sci. 2017, 8, 3260.
- 14For a recent comprehensive review on diastereodivergent enantioselective catalysis, see: M. Bihania, J. C.-G. Zhao, Adv. Synth. Catal. 2017, 359, 534.
- 15N. Kumagai, M. Shibasaki, Synthesis 2019, 185.
- 16
- 16aK. Weidner, N. Kumagai, M. Shibasaki, Angew. Chem. Int. Ed. 2014, 53, 6150; Angew. Chem. 2014, 126, 6264;
- 16bL. Brewitz, F. A. Arteaga, L. Yin, K. Alagiri, N. Kumagai, M. Shibasaki, J. Am. Chem. Soc. 2015, 137, 15929;
- 16cK. Weidner, Z. Sun, N. Kumagai, M. Shibasaki, Angew. Chem. Int. Ed. 2015, 54, 6236; Angew. Chem. 2015, 127, 6334;
- 16dZ. Liu, T. Takeuchi, R. Pluta, F. A. Arteaga, N. Kumagai, M. Shibasaki, Org. Lett. 2017, 19, 710;
- 16eB. Sun, P. V. Balaji, N. Kumagai, M. Shibasaki, J. Am. Chem. Soc. 2017, 139, 8295;
- 16fB. Sun, R. Pluta, N. Kumagai, M. Shibasaki, Org. Lett. 2018, 20, 526.
- 17
- 17aT. Tsuda, T. Yazawa, K. Watanabe, T. Fujii, T. Saegusa, J. Org. Chem. 1981, 46, 192;
- 17bE. M. Meyer, S. Gambarotta, C. Floriani, A. Chiesi-Villa, C. Guastinit, Organometallics 1989, 8, 1067;
- 17cM. Stollenz, F. Meyer, Organometallics 2012, 31, 7708.
- 18
- 18aH. Yamamoto, K. Futatsugi, Angew. Chem. Int. Ed. 2005, 44, 1924; Angew. Chem. 2005, 117, 1958;
- 18bD. H. Paull, C. J. Abraham, M. T. Scerba, E. Alden-Danforth, T. Lectka, Acc. Chem. Res. 2008, 41, 655;
- 18cN. Kumagai, M. Shibasaki, Angew. Chem. Int. Ed. 2011, 50, 4760; Angew. Chem. 2011, 123, 4856;
- 18dR. Peters, Cooperative Catalysis, Wiley-VCH, Weinheim, 2015.
10.1002/9783527681020 Google Scholar
- 19See the Supporting Information.
- 20The NMR study is summarized in the Supporting Information.
- 21A. G. Myers, B. H. Yang, H. Chen, L. McKinstry, D. J. Kopecky, J. L. Gleason, J. Am. Chem. Soc. 1997, 119, 6496.