Catalytic Asymmetric Synthesis of α-Arylpyrrolidines and Benzo-fused Nitrogen Heterocycles
Dr. Xi-Jie Dai
Department of Chemistry, Room 18-490, Massachusetts Institute of Technology, Cambridge, MA, 02139 USA
Search for more papers by this authorDr. Oliver D. Engl
Department of Chemistry, Room 18-490, Massachusetts Institute of Technology, Cambridge, MA, 02139 USA
Search for more papers by this authorDr. Thierry León
Department of Chemistry, Room 18-490, Massachusetts Institute of Technology, Cambridge, MA, 02139 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Stephen L. Buchwald
Department of Chemistry, Room 18-490, Massachusetts Institute of Technology, Cambridge, MA, 02139 USA
Search for more papers by this authorDr. Xi-Jie Dai
Department of Chemistry, Room 18-490, Massachusetts Institute of Technology, Cambridge, MA, 02139 USA
Search for more papers by this authorDr. Oliver D. Engl
Department of Chemistry, Room 18-490, Massachusetts Institute of Technology, Cambridge, MA, 02139 USA
Search for more papers by this authorDr. Thierry León
Department of Chemistry, Room 18-490, Massachusetts Institute of Technology, Cambridge, MA, 02139 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Stephen L. Buchwald
Department of Chemistry, Room 18-490, Massachusetts Institute of Technology, Cambridge, MA, 02139 USA
Search for more papers by this authorDedicated to Professor Ronald Raines on the occasion of his 60th birthday
Graphical Abstract
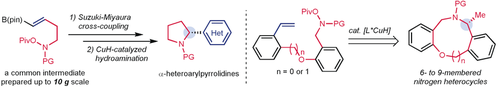
An enantioselective copper-catalyzed intramolecular hydroamination reaction can be used jointly with the Suzuki–Miyaura cross-coupling to yield a diverse array of α-arylpyrrolidine scaffolds that contain pharmaceutically relevant heteroarenes with excellent enantiomeric purity under mild conditions. Furthermore, this intramolecular hydroamination strategy is applicable to the asymmetric syntheses of six- to nine-membered benzo-fused nitrogen heterocycles.
Abstract
Herein, we report a practical two-step synthetic route to α-arylpyrrolidines through Suzuki–Miyaura cross-coupling and enantioselective copper-catalyzed intramolecular hydroamination reactions. The excellent stereoselectivity and broad scope for the transformation of substrates with pharmaceutically relevant heteroarenes render this method a practical and versatile approach for pyrrolidine synthesis. Additionally, this intramolecular hydroamination strategy facilitates the asymmetric synthesis of tetrahydroisoquinolines and medium-ring dibenzo-fused nitrogen heterocycles.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201814331-sup-0001-misc_information.pdf13.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aE. Vitaku, D. T. Smith, J. T. Njardarson, J. Med. Chem. 2014, 57, 10257–10274;
- 1bM. E. Welsch, S. A. Snyder, B. R. Stockwell, Curr. Opin. Chem. Biol. 2010, 14, 347–361;
- 1cR. D. Taylor, M. MacCoss, A. D. G. Lawson, J. Med. Chem. 2014, 57, 5845–5859.
- 2C. V. Galliford, K. A. Scheidt, Angew. Chem. Int. Ed. 2007, 46, 8748–8758; Angew. Chem. 2007, 119, 8902–8912.
- 3
- 3aJ. E. Sieser, M. T. Maloney, E. Chisowa, S. J. Brenek, S. Monfette, J. J. Salisbury, N. M. Do, R. A. Singer, Org. Process Res. Dev. 2018, 22, 527–534;
- 3bM. Baumann, I. R. Baxendale, C. Kuratli, S. V. Ley, R. E. Martin, J. Schneider, ACS Comb. Sci. 2011, 13, 405–413.
- 4
- 4aS. M. Banik, A. Levina, A. M. Hyde, E. N. Jacobsen, Science 2017, 358, 761–764;
- 4bS. E. Reisman, A. G. Doyle, E. N. Jacobsen, J. Am. Chem. Soc. 2008, 130, 7198–7199;
- 4cA. M. d'Albuquerque Rocha Gonsalves, M. E. da Silva Serra, M. R. Silva, A. M. Beja, J. A. Paixão, L. A. da Veiga, J. Mol. Catal. A 2001, 168, 53–59.
- 5F. Poordad, F. Felizarta, A. Asatryan, M. S. Sulkowski, R. W. Reindollar, C. S. Landis, S. C. Gordon, S. L. Flamm, M. W. Fried, D. E. Bernstein, C.-W. Lin, R. Liu, S. S. Lovell, T. I. Ng, J. Kort, F. J. Mensa, Hepatology 2017, 66, 389–397.
- 6M. Wang, S. Rule, P. L. Zinzani, A. Goy, O. Casasnovas, S. D. Smith, G. Damaj, J. Doorduijn, T. Lamy, F. Morschhauser, C. Panizo, B. Shah, A. Davies, R. Eek, J. Dupuis, E. Jacobsen, A. P. Kater, S. Le Gouill, L. Oberic, T. Robak, T. Covey, R. Dua, A. Hamdy, X. Huang, R. Izumi, P. Patel, W. Rothbaum, J. G. Slatter, W. Jurczak, Lancet 2018, 391, 659–667.
- 7
- 7aT. J. Ritchie, S. J. F. Macdonald, S. Peace, S. D. Pickett, C. N. Luscombe, MedChemComm 2012, 3, 1062–1069;
- 7bC. K. Prier, D. W. C. MacMillan, Chem. Sci. 2014, 5, 4173–4178.
- 8
- 8aD. C. Blakemore, L. Castro, I. Churcher, D. C. Rees, A. W. Thomas, D. M. Wilson, A. Wood, Nat. Chem. 2018, 10, 383–394;
- 8bP. S. Kutchukian, J. F. Dropinski, K. D. Dykstra, B. Li, D. A. DiRocco, E. C. Streckfuss, L.-C. Campeau, T. Cernak, P. Vachal, I. W. Davies, S. W. Krska, S. D. Dreher, Chem. Sci. 2016, 7, 2604–2613.
- 9
- 9aP. Jain, P. Verma, G. Xia, J.-Q. Yu, Nat. Chem. 2017, 9, 140–144;
- 9bC. J. Cordier, R. J. Lundgren, G. C. Fu, J. Am. Chem. Soc. 2013, 135, 10946–10949;
- 9cG.-H. Hou, J.-H. Xie, P.-C. Yan, Q.-L. Zhou, J. Am. Chem. Soc. 2009, 131, 1366–1367;
- 9dR. L. LaLonde, B. D. Sherry, E. J. Kang, F. D. Toste, J. Am. Chem. Soc. 2007, 129, 2452–2453;
- 9eM. C. Wood, D. C. Leitch, C. S. Yeung, J. A. Kozak, L. L. Schafer, Angew. Chem. Int. Ed. 2007, 46, 354–358; Angew. Chem. 2007, 119, 358–362;
- 9fA. R. Brown, C. Uyeda, C. A. Brotherton, E. N. Jacobsen, J. Am. Chem. Soc. 2013, 135, 6747–6749;
- 9gB. M. Trost, S. M. Silverman, J. Am. Chem. Soc. 2012, 134, 4941–4954;
- 9hN. S. Sheikh, D. Leonori, G. Barker, J. D. Firth, K. R. Campos, A. J. H. M. Meijer, P. O'Brien, I. Coldham, J. Am. Chem. Soc. 2012, 134, 5300–5308;
- 9iZ. Cui, H.-J. Yu, R.-F. Yang, W.-Y. Gao, C.-G. Feng, G.-Q. Lin, J. Am. Chem. Soc. 2011, 133, 12394–12397;
- 9jL. Rajender Reddy, S. G. Das, Y. Liu, M. Prashad, J. Org. Chem. 2010, 75, 2236–2246;
- 9kFor a recent review focus on the racemic synthesis of saturated N-heterocycles, see: C.-V. T. Vo, J. W. Bode, J. Org. Chem. 2014, 79, 2809–2815.
- 10
- 10aD. Hoppe, A. Carstens, T. Krämer, Angew. Chem. Int. Ed. Engl. 1990, 29, 1424–1425; Angew. Chem. 1990, 102, 1457–1459;
- 10bD. Hoppe, T. Hense, Angew. Chem. Int. Ed. Engl. 1997, 36, 2282–2316; Angew. Chem. 1997, 109, 2376–2410.
- 11
- 11aS. T. Kerrick, P. Beak, J. Am. Chem. Soc. 1991, 113, 9708–9710;
- 11bP. Beak, S. T. Kerrick, S. Wu, J. Chu, J. Am. Chem. Soc. 1994, 116, 3231–3239.
- 12Only one enantiomer of α-arylpyrrolidines is assessible using the commercially available (−)-sparteine. A (+)-sparteine surrogate was developed preceding the commercial availbility of (+)-sparteine: M. J. Dearden, C. R. Firkin, J.-P. R. Hermet, P. O'Brien, J. Am. Chem. Soc. 2002, 124, 11870–11871.
- 13
- 13aK. R. Campos, A. Klapars, J. H. Waldman, P. G. Dormer, C.-Y. Chen, J. Am. Chem. Soc. 2006, 128, 3538–3539;
- 13bA. Klapars, K. R. Campos, J. H. Waldman, D. Zewge, P. G. Dormer, C.-Y. Chen, J. Org. Chem. 2008, 73, 4986–4993.
- 14Y. Wang, X. Wen, X. Cui, X. P. Zhang, J. Am. Chem. Soc. 2018, 140, 4792–4796.
- 15
- 15aJ. A. Kozlowski, et al. (Merck Sharp & Dohme Corp.), World Pat., WO2012040923A1., 2012;
- 15bJ. Liu, et al. (Merck Sharp & Dohme Corp.), US Pat., 066238, 2016.
- 16Y. Miki, K. Hirano, T. Satoh, M. Miura, Angew. Chem. Int. Ed. 2013, 52, 10830–10834; Angew. Chem. 2013, 125, 11030–11034.
- 17S. Zhu, N. Niljianskul, S. L. Buchwald, J. Am. Chem. Soc. 2013, 135, 15746–15749.
- 18
- 18aS. Zhu, S. L. Buchwald, J. Am. Chem. Soc. 2014, 136, 15913–15916;
- 18bN. Niljianskul, S. Zhu, S. L. Buchwald, Angew. Chem. Int. Ed. 2015, 54, 1638–1641; Angew. Chem. 2015, 127, 1658–1661;
- 18cD. Niu, S. L. Buchwald, J. Am. Chem. Soc. 2015, 137, 9716–9721;
- 18dS. Zhu, N. Niljianskul, S. L. Buchwald, Nat. Chem. 2016, 8, 144–150;
- 18eS.-L. Shi, S. L. Buchwald, Nat. Chem. 2015, 7, 38–44;
- 18fS. Ichikawa, S. Zhu, S. L. Buchwald, Angew. Chem. Int. Ed. 2018, 57, 8714–8718; Angew. Chem. 2018, 130, 8850–8854; For a minireview on the enantioselective copper-catalyzed hydroamination reactions, see:
- 18gM. T. Pirnot, Y.-M. Wang, S. L. Buchwald, Angew. Chem. Int. Ed. 2016, 55, 48–57; Angew. Chem. 2016, 128, 48–57.
- 19H. Wang, J. C. Yang, S. L. Buchwald, J. Am. Chem. Soc. 2017, 139, 8428–8431.
- 20The absolute stereochemistry of α-arylpyrrolidines 2 a–k was assigned by analogy to that of 2 l (see Table 2).
- 21J. S. Bandar, M. T. Pirnot, S. L. Buchwald, J. Am. Chem. Soc. 2015, 137, 14812–14818.
- 22No further improvement was observed by the addition of Ph3P (entries 5 and 7). For the reactivity increase caused by adding Ph3P in another CuH system, see: B. H. Lipshutz, K. Noson, W. Chrisman, A. Lower, J. Am. Chem. Soc. 2003, 125, 8779–8789.
- 23The stereochemistry of β-substituted olefins has a significant influence on the reaction efficiency; see Refs. [19] and [21].
- 24N. C. Bruno, N. Niljianskul, S. L. Buchwald, J. Org. Chem. 2014, 79, 4161–4166.
- 25D. T. Ahneman, J. G. Estrada, S. Lin, S. D. Dreher, A. G. Doyle, Science 2018, 360, 186–190.
- 26Conditions developed to conduct aryl-aryl coupling of unprotected, nitrogen-rich heterocycles failed to improve efficiency in these azole cases: M. A. Düfert, K. L. Billingsley, S. L. Buchwald, J. Am. Chem. Soc. 2013, 135, 12877–12885.
- 27Although (R,R)-Ph-BPE provided the best results in Table 1, (S)-DTBM-SEGPHOS was chosen as the ligand to examine the scope of α-arylpyrrolidines because of cost, availability, and stability.
- 28It is currently unclear whether the erosion of selectivity is due to epimerization of the alkylcopper species before the C−N bond formation. For mechanistic discussions on enantioselective hydrocupration and subsequent epimerization relevant to the hydroboration of alkenes, see: Y. Xi, J. F. Hartwig, J. Am. Chem. Soc. 2017, 139, 12758–12772.
- 29Improved yields were also observed in synthesis of six- to nine-membered ring nitrogen heterocycles upon switching to (R,R)-Ph-BPE and DMMS.
- 30Our attempts to synthesize an enantioenriched α-phenylazetidine resulted in a 30 % yield of isolated product. 1H NMR analysis indicated the formation of several byproducts; see the Supporting Information for details.
- 31(R,R)-Ph-BPE and DMMS replaced (S)-DTBM-SEGPHOS and DEMS. However, under our standard hydroamination conditions (footnote [b], Table 2), formation of 2-phenylpiperidine 5 was not detected from 4 (footnote [b], Scheme 1) while 2-phenylpyrrolidine 2 a was isolated in 83 % yield from 1 ac.
- 32C.-V. T. Vo, M. U. Luescher, J. W. Bode, Nat. Chem. 2014, 6, 310–314.
- 33
- 33aJ. L. Kenwright, W. R. J. D. Galloway, D. T. Blackwell, A. Isidro-Llobet, J. Hodgkinson, L. Wortmann, S. D. Bowden, M. Welch, D. R. Spring, Chem. Eur. J. 2011, 17, 2981–2986;
- 33bP. Mestichelli, M. J. Scott, W. R. J. D. Galloway, J. Selwyn, J. S. Parker, D. R. Spring, Org. Lett. 2013, 15, 5448–5451;
- 33cA. Joncour, A. Décor, S. Thoret, A. Chiaroni, O. Baudoin, Angew. Chem. Int. Ed. 2006, 45, 4149–4152; Angew. Chem. 2006, 118, 4255–4258.
- 34The absolute stereochemistry of nitrogen heterocycles 2 m–o, 5, 7, 11, 12 was assigned by analogy to that of 10. See the Supporting Information for details.