Regiodivergent Hydroaminoalkylation of Alkynes and Allenes by a Combined Rhodium and Photoredox Catalytic System
Dr. Jun Zheng
Institut für Organische Chemie, Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg im Breisgau, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Bernhard Breit
Institut für Organische Chemie, Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg im Breisgau, Germany
Search for more papers by this authorDr. Jun Zheng
Institut für Organische Chemie, Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg im Breisgau, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Bernhard Breit
Institut für Organische Chemie, Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg im Breisgau, Germany
Search for more papers by this authorGraphical Abstract
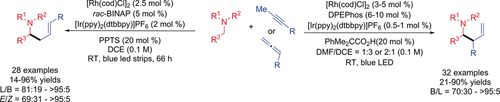
A direct cross-coupling of alkynes and allenes with amines to access α-allylated amines using a Rh/photoredox dual catalyst system was developed. Starting from easily available internal alkynes, tertiary amines, and secondary amines, various branched homoallylic amines were obtained with good to excellent yields and regioselectivity. In contrast, with a modified reaction conditions, the alkynes and terminal allenes could couple with various substituted N-aryl-tetrahydroisoquinolines smoothly affording (E)-linear homoallylic amines in good to excellent yields and regioselectivity.
Abstract
A rhodium/photoredox dual catalyzed regiodivergent α-allylation of amines is described. As an atom-economic and efficient method, alkynes and allenes are used as allylic electrophile surrogates in this novel protocol. With different reaction conditions, synthetically useful branched or linear homoallylic amines could be synthesized in good to excellent yields and regioselectivity. This straightforward strategy complements the traditional transition-metal catalyzed allylation reactions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201813646-sup-0001-misc_information.pdf19.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews on the application of allylic alkylation in total synthesis, see:
- 1aG. Helmchen, M. Ernst, G. Paradies, Pure Appl. Chem. 2004, 76, 495;
- 1bB. M. Trost, M. L. Crawley, Top. Organomet. Chem. 2012, 38, 321;
- 1cS. Arseniyadis, J. Fournier, S. Thangavelu, O. Lozano, S. Prevost, A. Archambeau, C. Menozzi, J. Cossy, Synlett 2013, 2350;
- 1dA. Y. Hong, B. M. Stoltz, Eur. J. Org. Chem. 2013, 2745;
- 1eK. C. Majumdar, B. Sinha, Synthesis 2013, 1271.
- 2B. M. Trost, Science 1991, 254, 1471.
- 3For recent reviews, see:
- 3aP. Koschker, B. Breit, Acc. Chem. Res. 2016, 49, 1524;
- 3bA. M. Haydl, B. Breit, T. Liang, M. J. Krische, Angew. Chem. Int. Ed. 2017, 56, 11312; Angew. Chem. 2017, 129, 11466.
- 4For selected reviews on transition-metal-catalyzed allylic substitution reaction, see:
- 4aJ. Tsuji, I. Minami, Acc. Chem. Res. 1987, 20, 140;
- 4bB. M. Trost, D. L. VanVranken, Chem. Rev. 1996, 96, 395;
- 4cB. M. Trost, M. L. Crawley, Chem. Rev. 2003, 103, 2921;
- 4dG. Helmchen, A. Dahnz, P. Dübon, M. Schelwies, R. Weihofen, Chem. Commun. 2007, 675;
- 4eZ. Lu, S.-M. Ma, Angew. Chem. Int. Ed. 2008, 47, 258; Angew. Chem. 2008, 120, 264;
- 4fJ. F. Hartwig, L. M. Stanley, Acc. Chem. Res. 2010, 43, 1461;
- 4gC.-X. Zhuo, C. Zheng, S.-L. You, Acc. Chem. Res. 2014, 47, 2558;
- 4hN. A. Butt, W.-B. Zhang, Chem. Soc. Rev. 2015, 44, 7929;
- 4iJ. C. Hethcox, S. E. Shockley, B. M. Stoltz, ACS Catal. 2016, 6, 6207; For selected reviews on allylic C−H functionalization, see:
- 4jS. E. Mann, L. Benhamou, T. D. Sheppard, Synthesis 2015, 47, 3079;
- 4kH.-M. Tang, X.-H. Huo, Q.-H. Meng, W.-B. Zhang, Acta Chim. Sin. 2016, 74, 219.
- 5For examples of C−C bond formation, see:
- 5aC. Li, B. Breit, J. Am. Chem. Soc. 2014, 136, 862;
- 5bT. M. Beck, B. Breit, Angew. Chem. Int. Ed. 2017, 56, 1903; Angew. Chem. 2017, 129, 1929;
- 5cT. M. Beck, B. Breit, Org. Lett. 2016, 18, 124;
- 5dF. A. Cruz, Z. Chen, S. I. Kurtoic, V. M. Dong, Chem. Commun. 2016, 52, 5836;
- 5eC. Li, C. P. Grugel, B. Breit, Chem. Commun. 2016, 52, 5840;
- 5fT. M. Beck, B. Breit, Eur. J. Org. Chem. 2016, 5839;
- 5gF. A. Cruz, V. M. Dong, J. Am. Chem. Soc. 2017, 139, 1029;
- 5hF. A. Cruz, Y. Zhu, Q. D. Tercenio, Z. Shen, V. M. Dong, J. Am. Chem. Soc. 2017, 139, 10641;
- 5iC. P. Grugel, B. Breit, Org. Lett. 2018, 20, 1066;
- 5jP. P. Bora, G.-J. Sun, W.-F. Zheng, Q. Kang, Chin. J. Chem. 2018, 36, 20;
- 5kC. P. Grugel, B. Breit, Chem. Eur. J. 2018, 24, 15223.
- 6For selected reviews on photoredox catalysis, see:
- 6aJ. M. R. Narayanam, C. R. J. Stephenson, Chem. Soc. Rev. 2011, 40, 102;
- 6bJ. Xuan, W.-J. Xiao, Angew. Chem. Int. Ed. 2012, 51, 6828; Angew. Chem. 2012, 124, 6934;
- 6cC. K. Prier, D. A. Rankic, D. W. C. MacMillan, Chem. Rev. 2013, 113, 5322;
- 6dD. M. Schultz, T. P. Yoon, Science 2014, 343, 1239176;
- 6eN. A. Romero, D. A. Nicewicz, Chem. Rev. 2016, 116, 10075;
- 6fK. Nakajima, Y. Miyake, Y. Nishibayashi, Acc. Chem. Res. 2016, 49, 1946;
- 6gJ. Xie, H. Jin, A. S. K. Hashmi, Chem. Soc. Rev. 2017, 46, 5193.
- 7For selected reviews on the merge of photoredox and transition metal catalysis, see:
- 7aK. L. Skubi, T. R. Blum, T. P. Yoon, Chem. Rev. 2016, 116, 10035;
- 7bJ. C. Tellis, C. B. Kelly, D. N. Primer, M. Jouffroy, N. R. Patel, G. A. Molander, Acc. Chem. Res. 2016, 49, 1429;
- 7cJ. Twilton, C. Le, P. Zhang, M. H. Shaw, R. W. Evans, D. W. C. MacMillan, Nat. Rev. Chem. 2017, 1, 0052;
- 7dE. Meggers, Angew. Chem. Int. Ed. 2017, 56, 5668; Angew. Chem. 2017, 129, 5760.
- 8
- 8aS. B. Lang, K. M. O'Nele, J. A. Tunge, J. Am. Chem. Soc. 2014, 136, 13606;
- 8bS. B. Lang, K. M. O'Nele, J. T. Douglas, J. A. Tunge, Chem. Eur. J. 2015, 21, 18589.
- 9J. Xuan, T.-T. Zeng, Z.-J. Feng, Q.-H. Deng, J.-R. Chen, L.-Q. Lu, W.-J. Xiao, H. Alper, Angew. Chem. Int. Ed. 2015, 54, 1625; Angew. Chem. 2015, 127, 1645.
- 10S. M. Thullen, T. Rovis, J. Am. Chem. Soc. 2017, 139, 15504.
- 11For other allylation reaction enabled by transition metal/photoredox dual catalysis, see:
- 11aJ. L. Schwarz, F. Schäfer, A. Tlahuext-Aca, L. Lückemeier, F. Glorius, J. Am. Chem. Soc. 2018, 140, 12705;
- 11bJ. K. Matsui, Á. Gutiérrez-Bonet, M. Rotella, R. Alam, O. Gutierrez, G. A. Molander, Angew. Chem. Int. Ed. 2018, 57, 15847–15851; Angew. Chem. 2018, 130, 16073–16077; During the writing of this manuscript a related work describing enantioselective allylic alkylation with 4-alkyl-1,4-dihydropyridines enabled by photoredox/Pd cocatalysis has appeared. H.-H. Zhang, J.-J. Zhao, S.-Y. Yu, J. Am. Chem. Soc. 2019, https://doi.org/10.1021/jacs.8b10766.
- 12Although in all the cases the distereoselectivity of the branched products are in 1:1 to 3.2:1, most of them could be separated by using column chromatography.
- 13H. Bartling, A. Eisenhofer, B. König, R. M. Gschwind, J. Am. Chem. Soc. 2016, 138, 11860.
- 14For the details, see the Supporting Information.
- 15For a mechanistic investigations of the rhodium catalyzed coupling of alkynes with carboxylic acids, see: U. Gellrich, A. Meissner, A. Steffani, M. Kaeny, H.-J. Drexler, D. Heller, D. A. Plattner, B. Breit, J. Am. Chem. Soc. 2014, 136, 1097.
- 16Although a cyclic voltammetry study of complex Rh1 gave a probable reduction potential of Rh1 (−1.93 V for [RhIII/RhI] vs. Fc/Fc+), the reduction potential of RhIII to RhII is still unknown. For more details on the potentials of RhIII complexes, see:
- 16aH. C.-Y. Bettega, J.-C. Moutet, J. Electroanal. Chem. 1994, 364, 271;
- 16bK. Murata, N. Numasawa, K. Shimomaki, J. Takaya, N. Iwasawa, Chem. Commun. 2017, 53, 3098.
- 17The oxidation of amine 1 a (Eox=0.79 V vs. SCE in MeCN) with IrIV species (E1/2red[IrIV/IrIII]=1.21 V vs. SCE in MeCN) should be easily accessible. In contrast, excited species *IrIII (E1/2red[*IrIII/IrII]=0.66 V vs. SCE in MeCN) is unlikely to oxidize amine 1 a, which is in agreement with Stern–Volmer quencher experiment. For the redox potential of Ir complex, see: M. S. Lowry, J. I. Goldsmith, J. D. Slinker, R. Rohl, R. A. Pascal, G. G. Malliaras, S. Bernhard, Chem. Mater. 2005, 17, 5712; Redox potential of amine 1 a, see: G. Wei, C. Zhang, F. Bures, X. Ye, C.-H. Tan, Z.-Y. Jiang, ACS Catal. 2016, 6, 3708.