A Noncovalent Fluorescence Turn-on Strategy for Hypoxia Imaging
Wen-Chao Geng
College of Chemistry, Key Laboratory of Functional Polymer Materials (Ministry of Education), State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorShaorui Jia
Key Laboratory of Bioactive Materials, Ministry of Education, College of Life Sciences, Nankai University, Tianjin, 300071 China
Search for more papers by this authorZhe Zheng
College of Chemistry, Key Laboratory of Functional Polymer Materials (Ministry of Education), State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorZhihao Li
College of Chemistry, Key Laboratory of Functional Polymer Materials (Ministry of Education), State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorProf. Dan Ding
Key Laboratory of Bioactive Materials, Ministry of Education, College of Life Sciences, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Dong-Sheng Guo
College of Chemistry, Key Laboratory of Functional Polymer Materials (Ministry of Education), State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorWen-Chao Geng
College of Chemistry, Key Laboratory of Functional Polymer Materials (Ministry of Education), State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorShaorui Jia
Key Laboratory of Bioactive Materials, Ministry of Education, College of Life Sciences, Nankai University, Tianjin, 300071 China
Search for more papers by this authorZhe Zheng
College of Chemistry, Key Laboratory of Functional Polymer Materials (Ministry of Education), State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorZhihao Li
College of Chemistry, Key Laboratory of Functional Polymer Materials (Ministry of Education), State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorProf. Dan Ding
Key Laboratory of Bioactive Materials, Ministry of Education, College of Life Sciences, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Dong-Sheng Guo
College of Chemistry, Key Laboratory of Functional Polymer Materials (Ministry of Education), State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorDedicated to Professor Yu Liu on the occasion of his 65th birthday
Graphical Abstract
Abstract
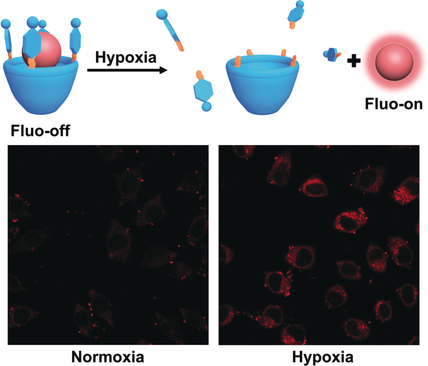
Hypoxia plays crucial roles in many diseases and is a central target for them. Present hypoxia imaging is restricted to the covalent approach, which needs tedious synthesis. In this work, a new supramolecular host–guest approach, based on the complexation of a hypoxia-responsive macrocycle with a commercial dye, is proposed. To exemplify the strategy, a carboxyl-modified azocalix[4]arene (CAC4A) was designed that binds to rhodamine 123 (Rho123) and quenches its fluorescence. The azo groups of CAC4A were selectively reduced under hypoxia, leading to the release of Rho123 and recovery of its fluorescence. The noncovalent strategy was validated through hypoxia imaging in living cells treated with the CAC4A–Rho123 reporter pair.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201813397-sup-0001-misc_information.pdf1.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. M. Brown, W. R. Wilson, Nat. Rev. Cancer 2004, 4, 437–447;
- 1bR. A. Gatenby, R. J. Gillies, Nat. Rev. Cancer 2008, 8, 56–61.
- 2Q. Chang, I. Jurisica, T. Do, D. W. Hedley, Cancer Res. 2011, 71, 3110–3120.
- 3B. A. Teicher, Cancer Metastasis Rev. 1994, 13, 139–168.
- 4Z. Huang, H. Xu, A. D. Meyers, A. I. Musani, L. Wang, R. Tagg, A. B. Barqawi, Y. K. Chen, Technol. Cancer Res. Treat. 2008, 7, 309–320.
- 5
- 5aL. Schito, G. L. Semenza, Trends Cancer 2016, 2, 758–770;
- 5bP. Vaupel, Oncologist 2008, 13, 21–26.
- 6
- 6aA. M. Jubb, F. M. Buffa, A. L. Harris, J. Cell. Mol. Med. 2010, 14, 18–29;
- 6bH. Minn, T. Gronroos, G. Komar, O. Eskola, K. Lehtio, J. Tuomela, M. Seppanen, O. Solin, Curr. Pharm. Des. 2008, 14, 2932–2942;
- 6cJ. G. Rajendran, K. R. Hendrickson, A. M. Spence, M. Muzi, K. A. Krohn, D. A. Mankoff, Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 44–53;
- 6dH. Shi, X. Ma, Q. Zhao, B. Liu, Q. Qu, Z. An, Y. Zhao, W. Huang, Adv. Funct. Mater. 2014, 24, 4823–4830;
- 6eY. Zeng, Y. Liu, J. Shang, J. Ma, R. Wang, L. Deng, Y. Guo, F. Zhong, M. Bai, S. Zhang, D. Wu, Plos One 2015, 10, e 0121293.
- 7S. Kizaka-Kondoh, M. Inoue, H. Harada, M. Hiraoka, Cancer Sci. 2003, 94, 1021–1028.
- 8
- 8aK. Mikami, M. Naito, A. Tomida, M. Yamada, T. Sirakusa, T. Tsuruo, Cancer Res. 1996, 56, 2823–2826;
- 8bY. Shen, Q. Zhang, X. Qian, Y. Yang, Anal. Chem. 2015, 87, 1274–1280.
- 9
- 9aA. Sharma, J. F. Arambula, S. Koo, R. Kumar, H. Singh, J. L. Sessler, J. S. Kim, Chem. Soc. Rev. 2019, https://doi.org/10.1039/C8CS00304A;
- 9bX. Li, N. Kwon, T. Guo, Z. Liu, J. Yoon, Angew. Chem. Int. Ed. 2018, 57, 11522–11531; Angew. Chem. 2018, 130, 11694–11704.
- 10
- 10aJ. Zhou, W. Shi, L.-H. Li, Q.-Y. Gong, X.-F. Wu, X.-H. Li, H.-M. Ma, Chem. Asian J. 2016, 11, 2719–2724;
- 10bJ. Zhang, H.-W. Liu, X.-X. Hu, J. Li, L.-H. Liang, X.-B. Zhang, W. Tan, Anal. Chem. 2015, 87, 11832–11839;
- 10cQ.-Q. Wan, X.-H. Gao, X.-Y. He, S.-M. Chen, Y.-C. Song, Q.-Y. Gong, X.-H. Li, H.-M. Ma, Chem. Asian J. 2014, 9, 2058–2062;
- 10dT. Guo, L. Cui, J. Shen, W. Zhu, Y. Xu, X. Qian, Chem. Commun. 2013, 49, 10820–10822;
- 10eL. Cui, Y. Zhong, W. Zhu, Y. Xu, Q. Du, X. Wang, X. Qian, Y. Xiao, Org. Lett. 2011, 13, 928–931;
- 10fH. S. Kim, A. Sharma, W. X. Ren, J. Han, J. S. Kim, Biomaterials 2018, 185, 63–72.
- 11
- 11aK. Kiyose, K. Hanaoka, D. Oushiki, T. Nakamura, M. Kajimura, M. Suematsu, H. Nishimatsu, T. Yamane, T. Terai, Y. Hirata, T. Nagano, J. Am. Chem. Soc. 2010, 132, 15846–15848;
- 11bA. Chevalier, W. Piao, K. Hanaoka, T. Nagano, P.-Y. Renard, A. Romieu, Methods Appl. Fluoresc. 2015, 3, 044004;
- 11cQ. Cai, T. Yu, W. Zhu, Y. Xu, X. Qian, Chem. Commun. 2015, 51, 14739–14741;
- 11dL. Sun, G. Li, X. Chen, Y. Chen, C. Jin, L. Ji, H. Chao, Sci. Rep. 2015, 5, 14837;
- 11eF. Perche, S. Biswas, T. Wang, L. Zhu, V. P. Torchilin, Angew. Chem. Int. Ed. 2014, 53, 3362–3366; Angew. Chem. 2014, 126, 3430–3434;
- 11fA. Popat, B. P. Ross, J. Liu, S. Jambhrunkar, F. Kleitz, S. Z. Qiao, Angew. Chem. Int. Ed. 2012, 51, 12486–12489; Angew. Chem. 2012, 124, 12654–12657;
- 11gP. Yuan, H. Zhang, L. Qian, X. Mao, S. Du, C. Yu, B. Peng, S. Q. Yao, Angew. Chem. Int. Ed. 2017, 56, 12481–12485; Angew. Chem. 2017, 129, 12655–12659.
- 12W. Piao, S. Tsuda, Y. Tanaka, S. Maeda, F. Liu, S. Takahashi, Y. Kushida, T. Komatsu, T. Ueno, T. Terai, T. Nakazawa, M. Uchiyama, K. Morokuma, T. Nagano, K. Hanaoka, Angew. Chem. Int. Ed. 2013, 52, 13028–13032; Angew. Chem. 2013, 125, 13266–13270.
- 13M. I. Uddin, S. M. Evans, J. R. Craft, L. J. Marnett, M. J. Uddin, A. Jayagopal, ACS Med. Chem. Lett. 2015, 6, 445–449.
- 14
- 14aX. Ma, Y. Zhao, Chem. Rev. 2015, 115, 7794–7839;
- 14bJ. Gao, J. Li, W.-C. Geng, F.-Y. Chen, X. Duan, Z. Zheng, D. Ding, D.-S. Guo, J. Am. Chem. Soc. 2018, 140, 4945–4953.
- 15A. Norouzy, Z. Azizi, W. M. Nau, Angew. Chem. Int. Ed. 2015, 54, 792–795; Angew. Chem. 2015, 127, 804–808.
- 16
- 16aV. Böhmer, Angew. Chem. Int. Ed. Engl. 1995, 34, 713–745; Angew. Chem. 1995, 107, 785–818;
- 16bM. A. Beatty, J. Borges-Gonzalez, N. J. Sinclair, A. T. Pye, F. Hof, J. Am. Chem. Soc. 2018, 140, 3500–3504.
- 17Y. Morita, T. Agawa, E. Nomura, H. Taniguchi, J. Org. Chem. 1992, 57, 3658–3662.
- 18V. Reshetnikov, S. Daum, C. Janko, W. Karawacka, R. Tietze, C. Alexiou, S. Paryzhak, T. Dumych, R. Bilyy, P. Tripal, B. Schmid, R. Palmisano, A. Mokhir, Angew. Chem. Int. Ed. 2018, 57, 11943–11946; Angew. Chem. 2018, 130, 12119–12122.
- 19
- 19aD.-S. Guo, H.-Q. Zhang, F. Ding, Y. Liu, Org. Biomol. Chem. 2012, 10, 1527–1536;
- 19bZ. Zheng, W.-C. Geng, J. Gao, Y.-Y. Wang, H. Sun, D.-S. Guo, Chem. Sci. 2018, 9, 2087–2091.
- 20
- 20aP. J. Stephens, F. J. Devlin, C. F. Chabalowski, M. J. Frisch, J. Phys. Chem. 1994, 98, 11623–11627;
- 20bA. D. Becke, J. Chem. Phys. 1993, 98, 5648–5652;
- 20cP. C. Hariharan, J. A. Pople, Theor. Chim. Acta 1973, 28, 213–222;
- 20dW. J. Hehre, R. Ditchfield, J. A. Pople, J. Chem. Phys. 1972, 56, 2257–2261.
- 21J. S. Murray, P. Politzer, WIREs Comput. Mol. Sci. 2011, 1, 153–163.
- 22C. Lefebvre, G. Rubez, H. Khartabil, J. C. Boisson, J. Contreras-Garcia, E. Henon, Phys. Chem. Chem. Phys. 2017, 19, 17928–17936.
- 23A. Chevalier, C. Mercier, L. Saurel, S. Orenga, P. Y. Renard, A. Romieu, Chem. Commun. 2013, 49, 8815–8817.
- 24J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer US, New York, 2006.
- 25
- 25aR. N. Dsouza, U. Pischel, W. M. Nau, Chem. Rev. 2011, 111, 7941–7980;
- 25bD.-S. Guo, V. D. Uzunova, X. Su, Y. Liu, W. M. Nau, Chem. Sci. 2011, 2, 1722–1734.
- 26A. Weller, Pure Appl. Chem. 1968, 16, 115–123.
- 27A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher, T. E. Rice, Chem. Rev. 1997, 97, 1515–1566.
- 28
- 28aG. Leriche, G. Budin, L. Brino, A. Wagner, Eur. J. Org. Chem. 2010, 4360–4364;
- 28bY. Y. Yang, M. Grammel, A. S. Raghavan, G. Charron, H. C. Hang, Chem. Biol. 2010, 17, 1212–1222.
- 29P. Verwilst, J. Han, J. Lee, S. Mun, H.-G. Kang, J. S. Kim, Biomaterials 2017, 115, 104–114.
- 30
- 30aJ. Rao, A. Khan, J. Am. Chem. Soc. 2013, 135, 14056–14059;
- 30bJ. Rao, C. Hottinger, A. Khan, J. Am. Chem. Soc. 2014, 136, 5872–5875;
- 30cR. Li, M. A. Bianchet, P. Talalay, L. M. Amzel, Proc. Natl. Acad. Sci. USA 1995, 92, 8846–8850.
- 31T. Mosmann, J. Immunol. Methods 1983, 65, 55–63.
- 32W.-C. Geng, H. Sun, D.-S. Guo, J. Inclusion Phenom. Macrocyclic Chem. 2018, 92, 1–79.