Kinetic Resolution of Tertiary Propargylic Alcohols by Enantioselective Cu−H-Catalyzed Si−O Coupling
Jan Seliger
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
Search for more papers by this authorDr. Xichang Dong
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Martin Oestreich
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
Search for more papers by this authorJan Seliger
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
Search for more papers by this authorDr. Xichang Dong
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Martin Oestreich
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin, Germany
Search for more papers by this authorGraphical Abstract
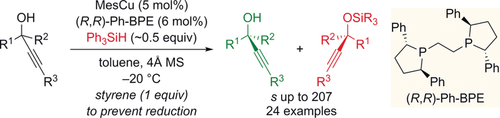
Matchmaker: The commercially available precatalyst system MesCu/(R,R)-Ph-BPE couples Ph3SiH with the fast-reacting enantiomer of racemic mixtures of tertiary propargylic alcohols. This non-enzymatic kinetic resolution provides access to synthetically valuable and versatile enantiomerically enriched tertiary propargylic alcohols and the corresponding silyl ethers (see scheme).
Abstract
A broad range of tertiary propargylic alcohols were kinetically resolved by catalyst-controlled enantioselective silylation. This non-enzymatic kinetic resolution is catalyzed by a Cu−H species and makes use of the commercially available precatalyst MesCu/(R,R)-Ph-BPE and a simple hydrosilane as the resolving reagent. Both alkyl,aryl- as well as dialkyl-substituted propargylic alcohols participate, and especially high selectivity factors are achieved when the alkyne terminus carries a TIPS group, which also enables facile post-functionalization in this position (s up to 207).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201813229-sup-0001-misc_information.pdf11 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aY.-L. Liu, X.-T. Lin, Adv. Synth. Catal., https://doi.org/10.1002/adsc.201801023;
- 1bM. Shibasaki, M. Kanai, Chem. Rev. 2008, 108, 2853–2873.
- 2
- 2aV. Bisai, V. K. Singh, Tetrahedron Lett. 2016, 57, 4771–4784;
- 2bT. Ohshima in Comprehensive Chirality, Vol. 4 (Eds.: ), Elsevier, New York, 2012, pp. 355–377;
- 2cB. M. Trost, A. H. Weiss, Adv. Synth. Catal. 2009, 351, 963–983.
- 3For selected examples of ligand-accelerated catalysis, see:
- 3aL. Tan, C.-y. Chen, R. D. Tillyer, E. J. J. Grabowski, P. J. Reider, Angew. Chem. Int. Ed. 1999, 38, 711–713;
10.1002/(SICI)1521-3773(19990301)38:5<711::AID-ANIE711>3.0.CO;2-W CAS PubMed Web of Science® Google ScholarAngew. Chem. 1999, 111, 724–727;
- 3bP. G. Cozzi, Angew. Chem. Int. Ed. 2003, 42, 2895–2898; Angew. Chem. 2003, 115, 3001–3004;
- 3cS. Kotani, K. Kukita, K. Tanaka, T. Ichibakase, M. Nakajima, J. Org. Chem. 2014, 79, 4817–4825.
- 4For selected examples of metal-catalyzed alkynylations of unactivated ketones, see:
- 4aG. Lu, X. Li, X. Jia, W. L. Chan, A. S. C. Chan, Angew. Chem. Int. Ed. 2003, 42, 5057–5058; Angew. Chem. 2003, 115, 5211–5212;
- 4bY. Zhou, R. Wang, Z. Xu, W. Yan, L. Liu, Y. Kang, Z. Han, Org. Lett. 2004, 6, 4147–4149;
- 4cL. Liu, R. Wang, Y.-F. Kang, C. Chen, Z.-Q. Xu, Y.-F. Zhou, M. Ni, H.-Q. Cai, M.-Z. Gong, J. Org. Chem. 2005, 70, 1084–1086;
- 4dJ.-i. Ito, S. Ubukata, S. Muraoka, H. Nishiyama, Chem. Eur. J. 2016, 22, 16801–16804;
- 4eA. M. Cook, C. Wolf, Angew. Chem. Int. Ed. 2016, 55, 2929–2933; Angew. Chem. 2016, 128, 2982–2986;
- 4fY. Zheng, Y. Tan, K. Harms, M. Marsch, R. Riedel, L. Zhang, E. Meggers, J. Am. Chem. Soc. 2017, 139, 4322–4325.
- 5For enantioselective additions to ynones, see:
- 5aS. Lou, P. N. Moquist, S. E. Schaus, J. Am. Chem. Soc. 2006, 128, 12660–12661 (allylation; one example);
- 5bD. K. Friel, M. L. Snapper, A. H. Hoveyda, J. Am. Chem. Soc. 2008, 130, 9942–9951 (with a pyridyl directing group);
- 5cH. Kawai, K. Tachi, E. Tokunaga, M. Shiro, N. Shibata, Org. Lett. 2010, 12, 5104–5107 (trifluoromethylation only).
- 6For substrate-controlled strategies, see:
- 6aM. I. Antczak, F. Cai, J. M. Ready, Org. Lett. 2011, 13, 184–187;
- 6bR. I. Rodríguez, E. Ramírez, F. Yuste, R. Sánchez-Obregón, J. Alemán, J. Org. Chem. 2018, 83, 1940–1947.
- 7
- 7aE. Vedejs, M. Jure, Angew. Chem. Int. Ed. 2005, 44, 3974–4001; Angew. Chem. 2005, 117, 4040–4069;
- 7bH. B. Kagan, J. C. Fiaud in Topics in Stereochemistry, Vol. 18 (Eds.: ), Wiley, New York, 1988, pp. 249–330.
10.1002/9780470147276.ch4 Google Scholar
- 8The field of enzymatic kinetic resolution to furnish enantioenriched tertiary alcohols was especially advanced by Bornscheuer and co-workers. For an authoritative review, see:
- 8aR. Kourist, P. Domínguez de María, U. T. Bornscheuer, ChemBioChem 2008, 9, 491–498; for original work, see for example:
- 8bS. Bartsch, R. Kourist, U. T. Bornscheuer, Angew. Chem. Int. Ed. 2008, 47, 1508–1511; Angew. Chem. 2008, 120, 1531–1534.
- 9For selected examples of non-enzymatic kinetic resolutions furnishing tertiary alcohols, see:
- 9aE. R. Jarvo, C. A. Evans, G. T. Copeland, S. J. Miller, J. Org. Chem. 2001, 66, 5522–5527;
- 9bT. G. Driver, J. R. Harris, K. A. Woerpel, J. Am. Chem. Soc. 2007, 129, 3836–3837;
- 9cR. Shintani, K. Takatsu, T. Hayashi, Org. Lett. 2008, 10, 1191–1193;
- 9dB. Karatas, S. Rendler, R. Fröhlich, M. Oestreich, Org. Biomol. Chem. 2008, 6, 1435–1440;
- 9eZ. Li, V. Boyarskikh, J. H. Hansen, J. Autschbach, D. G. Musaev, H. M. L. Davies, J. Am. Chem. Soc. 2012, 134, 15497–15504;
- 9fS. Lu, S. B. Poh, W.-Y. Siau, Y. Zhao, Angew. Chem. Int. Ed. 2013, 52, 1731–1734; Angew. Chem. 2013, 125, 1775–1778;
- 9gJ. L. Olivares-Romero, Z. Li, H. Yamamoto, J. Am. Chem. Soc. 2013, 135, 3411–3413;
- 9hM. D. Greenhalgh, S. M. Smith, D. M. Walden, J. E. Taylor, Z. Brice, E. R. T. Robinson, C. Fallan, D. B. Cordes, A. M. Z. Slawin, H. C. Richardson, M. A. Grove, P. H.-Y. Cheong, A. D. Smith, Angew. Chem. Int. Ed. 2018, 57, 3200–3206; Angew. Chem. 2018, 130, 3254–3260;
- 9iW. Zhang, S. Ma, Chem. Commun. 2018, 54, 6064–6067.
- 10
- 10aS. Rendler, G. Auer, M. Oestreich, Angew. Chem. Int. Ed. 2005, 44, 7620–7624; Angew. Chem. 2005, 117, 7793–7797;
- 10bS. Rendler, O. Plefka, B. Karatas, G. Auer, R. Fröhlich, C. Mück-Lichtenfeld, S. Grimme, M. Oestreich, Chem. Eur. J. 2008, 14, 11512–11528.
- 11A. Weickgenannt, M. Mewald, T. W. T. Muesmann, M. Oestreich, Angew. Chem. Int. Ed. 2010, 49, 2223–2226; Angew. Chem. 2010, 122, 2269–2272.
- 12C. Lorenz, U. Schubert, Chem. Ber. 1995, 128, 1267–1269.
- 13X. Dong, A. Weickgenannt, M. Oestreich, Nat. Commun. 2017, 8, 15547.
- 14For reviews on enantioselective alcohol silylation, see:
- 14aL.-W. Xu, Y. Chen, Y. Lu, Angew. Chem. Int. Ed. 2015, 54, 9456–9466; Angew. Chem. 2015, 127, 9590–9601;
- 14bA. Weickgenannt, M. Mewald, M. Oestreich, Org. Biomol. Chem. 2010, 8, 1497–1504; selected examples of other approaches include:
- 14cT. Isobe, K. Fukuda, Y. Araki, T. Ishikawa, Chem. Commun. 2001, 243–244;
- 14dY. Zhao, A. W. Mitra, A. H. Hoveyda, M. L. Snapper, Angew. Chem. Int. Ed. 2007, 46, 8471–8474; Angew. Chem. 2007, 119, 8623–8626;
- 14eC. I. Sheppard, J. L. Taylor, S. L. Wiskur, Org. Lett. 2011, 13, 3794–3797;
- 14fA. D. Worthy, X. Sun, K. L. Tan, J. Am. Chem. Soc. 2012, 134, 7321–7324;
- 14gS. Y. Park, J.-W. Lee, C. E. Song, Nat. Commun. 2015, 6, 7512.
- 15The occurrence of this side reaction was confirmed under the optimized reaction setup (Table 1, entry 8) in the absence of styrene. Partial semireduction of the C≡C triple bond in 1 a led to the corresponding R,Z-configured allylic alcohol; (S)-3 ah, (R)-1 a, and the allylic alcohol were formed in a ratio of 55:26:19. Interestingly, the silyl ether derived from this allylic alcohol was not detected, indicating that the silyl ether (S)-3 ah is not reduced and that the allylic alcohol is not silylated under these reaction conditions (see the Supporting Information for details).
- 16It should be emphasized that here and elsewhere the limited accuracy of the analytical tools used to determine enantiomeric excesses and conversions translate into imprecise selectivity factors. Consequently, the reported values merely approximate the order of magnitude. For a discussion of various error sources, see: M. D. Greenhalgh, J. E. Taylor, A. D. Smith, Tetrahedron 2018, 74, 5554–5560.
- 17In analogy to our earlier reagent-controlled strategy (Scheme 2, top),[9d] both alkyl- and aryl-substituted silolane and 2,3-dihydrobenzosilole derivatives were tested, but with limited success (see the Supporting Information for details).
- 18For an example of a transition-metal-promoted β-carbon elimination of propargylic alcohols, see: T. Nishimura, H. Araki, Y. Maeda, S. Uemura, Org. Lett. 2003, 5, 2997–2999.
- 19Conversions were determined by GLC analysis using docosane as an internal standard and calculated according to conversion=eeunreacted alcohol/(eesilyl ether+eeunreacted alcohol).
- 20
- 20aEnantiomeric excesses of the reisolated alcohols were determined by HPLC analysis on chiral stationary phases. Enantiomeric excesses of the silyl ethers were determined by HPLC analysis on chiral stationary phases after cleavage of the Si−O bond.
- 20bEnantiomeric excesses were determined by HPLC analysis on chiral stationary phases after transforming the slow-reacting alcohols and the deprotected fast-reacting alcohols into the corresponding 4-nitrobenzoyl esters.
- 21s=ln[(1−C)(1−ee)]/ln[(1−C)(1+ee)], where ee=eeunreacted alcohol/100 and C=conversion/100.
- 22In the context of a Cu−H-catalyzed hydroamination of alkynes, Shi and Buchwald found that a dialkyl-substituted internal triple bond did not undergo hydrocupration in the presence of an aryl-substituted triple bond; see: S.-L. Shi, S. L. Buchwald, Nat. Chem. 2015, 7, 38–44.