PdII-Catalyzed Enantioselective C(sp3)−H Activation/Cross-Coupling Reactions of Free Carboxylic Acids
Liang Hu
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorPeng-Xiang Shen
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorDr. Qian Shao
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorDr. Kai Hong
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorDr. Jennifer X. Qiao
Discovery Chemistry, Bristol-Myers Squibb Company, P.O. Box 4000, Princeton, NJ, 08543 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Jin-Quan Yu
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorLiang Hu
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorPeng-Xiang Shen
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorDr. Qian Shao
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorDr. Kai Hong
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorDr. Jennifer X. Qiao
Discovery Chemistry, Bristol-Myers Squibb Company, P.O. Box 4000, Princeton, NJ, 08543 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Jin-Quan Yu
Department of Chemistry, The Scripps Research Institute (TSRI), 10550 North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorGraphical Abstract
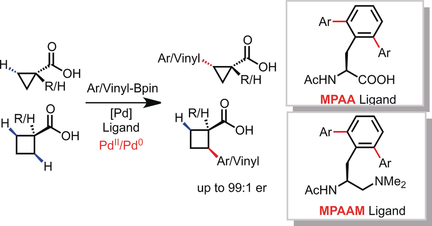
Worth protecting: PdII-catalyzed enantioselective C(sp3)−H cross-coupling of free carboxylic acids with organoborons has been realized using either mono-protected amino acid (MPAA) ligands or mono-protected aminoethyl amine (MPAAM) ligands. A diverse range of aryl- and vinyl-boron reagents can be used as coupling partners to provide chiral carboxylic acids.
Abstract
PdII-catalyzed enantioselective C(sp3)−H cross-coupling of free carboxylic acids with organoborons has been realized using either mono-protected amino acid (MPAA) ligands or mono-protected aminoethyl amine (MPAAM) ligands. A diverse range of aryl- and vinyl-boron reagents can be used as coupling partners to provide chiral carboxylic acids. This reaction provides an alternative approach to the enantioselective synthesis of cyclopropanecarboxylic acids and cyclobutanecarboxylic acids containing α-chiral tertiary and quaternary stereocenters. The utility of this reaction was further demonstrated by converting the carboxylic acid into cyclopropyl amine without loss of optical activity.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201813055-sup-0001-misc_information.pdf9.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews of enantioselective C−H functionalization, see:
- 1aR. Giri, B.-F. Shi, K. M. Engle, N. Maugel, J.-Q. Yu, Chem. Soc. Rev. 2009, 38, 3242;
- 1bC. G. Newton, S.-G. Wang, C. C. Oliveira, N. Cramer, Chem. Rev. 2017, 117, 8908;
- 1cT. G. Saint-Denis, R.-Y. Zhu, G. Chen, Q.-F. Wu, J.-Q. Yu, Science 2018, 359, eaao 4798.
- 2For the early observation of enantioselective C(sp3)−H functionalization using mono-N-protected amino acid ligands (MPAA) and pyridine directing group, see: B.-F. Shi, N. Maugel, Y.-H. Zhang, J.-Q. Yu, Angew. Chem. Int. Ed. 2008, 47, 4882; Angew. Chem. 2008, 120, 4960.
- 3
- 3aM. Wasa, K. M. Engle, D. W. Lin, E. J. Yoo, J.-Q. Yu, J. Am. Chem. Soc. 2011, 133, 19598;
- 3bK. S. L. Chan, H.-Y. Fu, J.-Q. Yu, J. Am. Chem. Soc. 2015, 137, 2042;
- 3cG. Chen, W. Gong, Z. Zhuang, M. S. Andra, Y.-Q. Chen, X. Hong, Y.-F. Yang, T. Liu, K. N. Houk, J.-Q. Yu, Science 2016, 353, 1023;
- 3dQ.-F. Wu, P.-X. Shen, J. He, X.-B. Wang, F. Zhang, Q. Shao, R.-Y. Zhu, C. Mapelli, J. X. Qiao, M. A. Poss, J.-Q. Yu, Science 2017, 355, 499;
- 3eQ. Shao, Q.-F. Wu, J. He, J.-Q. Yu, J. Am. Chem. Soc. 2018, 140, 5322.
- 4For selected Pd0-catalyzed intramolecular enantioselective C−H functionalization, see:
- 4aM. Nakanishi, D. Katayev, C. Besnard, E. P. Kündig, Angew. Chem. Int. Ed. 2011, 50, 7438; Angew. Chem. 2011, 123, 7576;
- 4bS. Anas, A. Cordi, H. B. Kagan, Chem. Commun. 2011, 47, 11483;
- 4cT. Saget, N. Cramer, Angew. Chem. Int. Ed. 2012, 51, 12842; Angew. Chem. 2012, 124, 13014;
- 4dN. Martin, C. Pierre, M. Davi, R. Jazzar, O. Baudoin, Chem. Eur. J. 2012, 18, 4480;
- 4eJ. Pedroni, N. Cramer, Angew. Chem. Int. Ed. 2015, 54, 11826; Angew. Chem. 2015, 127, 11992;
- 4fC. L. Ladd, A. B. Charette, Org. Lett. 2016, 18, 6046.
- 5For PdII-catalyzed enantioselective C−H functionalization using phosphoric acids/amides ligands, see:
- 5aS.-B. Yan, S. Zhang, W.-L. Duan, Org. Lett. 2015, 17, 2458;
- 5bH. Wang, H.-R. Tong, G. He, G. Chen, Angew. Chem. Int. Ed. 2016, 55, 15387; Angew. Chem. 2016, 128, 15613;
- 5cP. Jain, P. Verma, G. Xia, J.-Q. Yu, Nat. Chem. 2017, 9, 140.
- 6P. S. Baran, T. J. Maimone, J. M. Richter, Nature 2007, 446, 404.
- 7P.-X. Shen, L. Hu, Q. Shao, K. Hong, J.-Q. Yu, J. Am. Chem. Soc. 2018, 140, 6545.
- 8For selected reviews and reactions of asymmetric cyclopropanation, see:
- 8aM. P. Doyle, M. A. McKervey, T. Ye, Modern Catalytic Methods for Organic Synthesis with Diazo Compounds: From Cyclopropanes to Ylides, Wiley, New York, 1998;
- 8bM. P. Doyle, M. N. Protopopova, Tetrahedron 1998, 54, 7919;
- 8cS. E. Denmark, G. Beutner, In Cycloaddition Reactions in Organic Synthesis (Eds.: ), Wiley-VCH, Weinheim, 2002, pp. 85ff;
- 8dH. Lebel, J.-F. Marcoux, C. Molinaro, A. B. Charette, Chem. Rev. 2003, 103, 977;
- 8eY. Lou, M. Horikawa, R. A. Kloster, N. A. Hawryluk, E. J. Corey, J. Am. Chem. Soc. 2004, 126, 8916;
- 8fS. R. Goudreau, A. B. Charette, J. Am. Chem. Soc. 2009, 131, 15633;
- 8gS. Zhu, X. Cui, X. P. Zhang, Eur. J. Inorg. Chem. 2012, 430;
- 8hY. Wang, X. Wen, X. Cui, L. Wojtas, X. P. Zhang, J. Am. Chem. Soc. 2017, 139, 1049.
- 9For RhI-catalyzed intramolecular enantioselective C−H functionalization of cyclopropanes, see: T. Lee, J. F. Hartwig, Angew. Chem. Int. Ed. 2016, 55, 8723; Angew. Chem. 2016, 128, 8865.
- 10K.-J. Xiao, D. W. Lin, M. Miura, R.-Y. Zhu, W. Gong, M. Wasa, J.-Q. Yu, J. Am. Chem. Soc. 2014, 136, 8138.
- 11For reviews of the synthesis of enantiopure cyclobutane derivatives, see:
- 11aE. Lee-Ruff, G. Mladenova, Chem. Rev. 2003, 103, 1449;
- 11bF. Secci, A. Frongia, P. P. Piras, Molecules 2013, 18, 15541.
- 12For selected examples of Pd-catalyzed enantioselective C−H functionalization of cyclobutanes, see:
- 12aJ. He, Q. Shao, Q.-F. Wu, J.-Q. Yu, J. Am. Chem. Soc. 2017, 139, 3344;
- 12bQ.-F. Wu, X.-B. Wang, P.-X. Shen, J.-Q. Yu, ACS Catal. 2018, 8, 2577.
- 13M. H. Shaw, N. G. McCreanor, W. G. Whittingham, J. F. Bower, J. Am. Chem. Soc. 2015, 137, 463.