Nylon Intermediates from Bio-Based Levulinic Acid
Annemarie Marckwordt
Leibniz Institut für Katalyse e. V. an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
Search for more papers by this authorFatima El Ouahabi
Leibniz Institut für Katalyse e. V. an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
Search for more papers by this authorDr. Hadis Amani
Leibniz Institut für Katalyse e. V. an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
Search for more papers by this authorDr. Sergey Tin
Leibniz Institut für Katalyse e. V. an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
Search for more papers by this authorCorresponding Author
Dr. Narayana V. Kalevaru
Leibniz Institut für Katalyse e. V. an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
Search for more papers by this authorProf. Dr. Paul C. J. Kamer
Leibniz Institut für Katalyse e. V. an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
Search for more papers by this authorDr. Sebastian Wohlrab
Leibniz Institut für Katalyse e. V. an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Johannes G. de Vries
Leibniz Institut für Katalyse e. V. an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
Search for more papers by this authorAnnemarie Marckwordt
Leibniz Institut für Katalyse e. V. an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
Search for more papers by this authorFatima El Ouahabi
Leibniz Institut für Katalyse e. V. an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
Search for more papers by this authorDr. Hadis Amani
Leibniz Institut für Katalyse e. V. an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
Search for more papers by this authorDr. Sergey Tin
Leibniz Institut für Katalyse e. V. an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
Search for more papers by this authorCorresponding Author
Dr. Narayana V. Kalevaru
Leibniz Institut für Katalyse e. V. an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
Search for more papers by this authorProf. Dr. Paul C. J. Kamer
Leibniz Institut für Katalyse e. V. an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
Search for more papers by this authorDr. Sebastian Wohlrab
Leibniz Institut für Katalyse e. V. an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Johannes G. de Vries
Leibniz Institut für Katalyse e. V. an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
Search for more papers by this authorGraphical Abstract
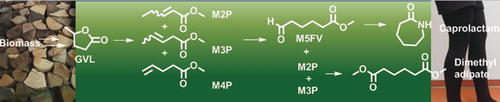
Green nylon: Use of 25 % ZrO2/SiO2 as catalyst allows the gas-phase ring-opening of bio-based γ-valerolactone with methanol to a mixture of methyl pentenoates containing 81 % of the 4-isomer, which could be selectively hydroformylated from the mixture to methyl 5-formyl-valerate, an intermediate for ϵ-caprolactam. The remaining isomers were converted into dimethyl adipate.
Abstract
Use of ZrO2/SiO2 as a solid acid catalyst in the ring-opening of biobased γ-valerolactone with methanol in the gas phase leads to mixtures of methyl 2-, 3-, and 4-pentenoate (MP) in over 95 % selectivity, containing a surprising 81 % of M4P. This process allows the application of a selective hydroformylation to this mixture to convert M4P into methyl 5-formyl-valerate (M5FV) with 90 % selectivity. The other isomers remain unreacted. Reductive amination of M5FV and ring-closure to ϵ-caprolactam in excellent yield had been reported before. The remaining mixture of 2- and 3-MP was subjected to an isomerising methoxycarbonylation to dimethyl adipate in 91 % yield.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201812954-sup-0001-misc_information.pdf489.5 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1P. Schlack (I.G. Farbenindustrie AG), US 2,241,321, 1941.
- 2J. Tinge, M. Groothaert, H. op het Veld, J. Ritz, H. Fuchs, H. Kieczka, W. C. Moran, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2018, Web-version
10.1002/14356007.a05_031.pub3 Google Scholarhttps://doi.org/10.1002/14356007.a05_031.pub3.
- 3
- 3aE. Drent, W. W. Jager (Shell Internationale Research Maatschappy B.V.), EP0577205, 1994;
- 3bO. E. Sielcken, H. Hovenkamp (DSM N.V. and E.I. Dupont de Nemours and Company), WO 95/06027, 1995;
- 3cE. Drent, W. Jager, O. E. Sielcken, I. Toth (DSM N.V.), WO 2002026690, 2002;
- 3dM. Beller, A. Krotz, W. Baumann, Adv. Synth. Catal. 2002, 344, 517–524.
- 4
- 4aE. Billig, A. G. Abatjoglou, D. R. Bryant (Union Carbide Corporation), US 4,769,498, 1988;
- 4bE. Billig, A. G. Abatjoglou, D. R. Bryant (Union Carbide Corporation), US 4,748,261, 1988.
- 5
- 5aM. Roeper, P. M. Lorz, D. Koeffer (BASF AG), EP 0 556 681, 1993;
- 5bC. B. Hansen, J. G. de Vries (DSM N.V. and E.I. Dupont de Nemours and Company), WO 9518089, 1995;
- 5cO. J. Gelling, P. C. Borman (DSM N.V. and Dupont de Nemours and Company), WO 98/19990, 1999.
- 6H. W. Schneider, R. Kummer, D. Zimmerling (BASF AG), EP 0 157 311, 1989.
- 7A. J. J. M. Teunissen, J. G. de Vries, O. J. Gelling, C. Lensink (DSM N.V.), WO 94/026688, 1994.
- 8R. P. M. Guit, W. Buis (DSM N.V.), EP0826666, 1998.
- 9
- 9aK. M. Draths, J. W. Frost, J. Am. Chem. Soc. 1994, 116, 399–400;
- 9bJ. Frost (Michigan State University), US20070149777, 2004;
- 9cT. R. Boussie, E. L. Dias, Z. M. Fresco, V. J. Murphy (Rennovia, Inc.), WO2010144873, 2010;
- 9dT. Buntara, S. Noel, P. H. Phua, I. Melián-Cabrera, J. G. de Vries, H. J. Heeres, Angew. Chem. Int. Ed. 2011, 50, 7083–7087; Angew. Chem. 2011, 123, 7221–7225;
- 9eD. D. Wu, Z. Chen, Z. B. Jia, L. Shuai, Sci. China Chem. 2012, 55, 380–385;
- 9fM. Shiramizu, F. D. Toste, Angew. Chem. Int. Ed. 2013, 52, 12905–12909; Angew. Chem. 2013, 125, 13143–13147;
- 9gX. Li, D. Wu, T. Lu, G. Yi, H. Su, Y. Zhang, Angew. Chem. Int. Ed. 2014, 53, 4200–4204; Angew. Chem. 2014, 126, 4284–4288;
- 9hS. Raoufmoghaddam, M. T. M. Rood, F. K. W. Buijze, E. Drent, E. Bouwman, ChemSusChem 2014, 7, 1984–1990;
- 9iR. Beerthuis, G. Rothenberg, N. R. Shiju, Green Chem. 2015, 17, 1341–1361;
- 9jT. R. Boussie, G. M. Diamond, E. Dias, V. Murphy in Chemicals and Fuels from Bio-Based Building Blocks (Eds.: ), Wiley-VCH, Weinheim, 2016, pp. 151–172;
10.1002/9783527698202.ch6 Google Scholar
- 9kH. Zhang, X. Li, X. Su, E. L. Ang, Y. Zhang, H. Zhao, ChemCatChem 2016, 8, 1500–1506;
- 9lJ. D. Nobbs, N. Z. B. Zainal, J. Tan, E. Drent, L. P. Stubbs, C. Li, S. C. Y. Lim, D. G. A. Kumbang, M. van Meurs, ChemistrySelect 2016, 1, 539–544;
- 9mY. Yang, X. Wei, F. Zenga, L. Deng, Green Chem. 2016, 18, 691–694;
- 9nR. T. Larson, A. Samant, J. Chen, W. Lee, M. A. Bohn, D. M. Ohlmann, S. J. Zuend, F. D. Toste, J. Am. Chem. Soc. 2017, 139, 14001–14004;
- 9oM. J. Gilkey, R. Balakumar, D. G. Vlachos, B. Xu, Catal. Sci. Technol. 2018, 8, 2661–2671.
- 10N. M. van der Velden, M. K. Patel, J. G. Vogtländer, Int. J. Life Cycle Assess. 2014, 19, 331–356.
- 11Top Value-Added Chemicals from Biomass Vol. I-Results of Screening for Potential Candidates from Sugars and Synthesis Gas (Eds.: T. Werpy, G. Petersen), U.S. Department of Energy (DOE) by the National Renewable Energy Laboratory a DOE national Laboratory, 2004.
- 12F. D. Pileidis, M.-M. Titirici, ChemSusChem 2016, 9, 562–582.
- 13D. J. Hayes, S. W. Fitzpatrick, M. H. B. Hayes, J. R. H. Ross in Biorefineries-Industrial Processes and Products: Status Quo and Future Directions, Vol. 1 (Eds.: ), Wiley, Weinheim, 2006, pp. 139–163.
- 14A. M. Raspolli Galletti, C. Antonetti, V. De Luise, D. Licursi, N. Nassi, BioResources 2012, 7, 1824–1835.
- 15http://www.gfbiochemicals.com.
- 16W. R. H. Wright, R. Palkovits, ChemSusChem 2012, 5, 1657–1667.
- 17U. Omoruyi, S. Page, J. Hallett, P. W. Miller, ChemSusChem 2016, 9, 2037–2047.
- 18
- 18aW. Hoelderich, F. Näumann, R. Fischer (BASF AG), US 5,144,061, 1992;
- 18bR. Fischer, U. Vagi (BASF AG), US 4, 740,613, 1988;
- 18cA. M. C. F. Castelijns (DSM IP Assetts B.V.), US 2013079548, 2013.
- 19
- 19aR. J. Haan, J.-P. Lange (Shell Internationale Research Maatschappij B.V.), WO2005/058793, 2005;
- 19bJ. P. Lange, J. Z. Vestering, R. J. Haan, Chem. Commun. 2007, 3488–3490;
- 19cF.-X. Zeng, H.-F. Liu, L. Deng, B. Liao, H. Pang, Q.-X. Guo, ChemSusChem 2013, 6, 600–603.
- 20L. E. Manzer (E.I. Dupont du Nemours and Company), WO04007421, 2004.
- 21The formation of stable rhodium enolates has also been observed in the hydrofomylation of methyl vinylketone: E. Walczuk, P. C. J. Kamer, P. W. N. M. van Leeuwen, Angew. Chem. Int. Ed. 2003, 42, 4665–4669; Angew. Chem. 2003, 115, 4813–4817.
- 22Z. J. Brentzel, M. R. Ball, J. A. Dumesic, Catal. Lett. 2018, 148, 3072–3081.
- 23BASF and DSM have already shown that 5MFV and 4MFV can be separated by distillation:
- 23aG. Achhammer, M. Röper (BASF AG), WO 97/06126, 1997;
- 23bO. J. Gelling, P. C. Borman (DSM NV and E.I. Dupont de Nemours and Co), 98/19990, 1998.
- 24
- 24aJ. G. de Vries, N. Sereinig, E. W. M. van de Vondervoort, M. C. C. Janssen (DSM IP Assets B.V.), WO 2012/131027, 2013;
- 24bJ. G. de Vries, Chem. Rec. 2016, 16, 2787–2800.
- 25C. Jiménez-Rodriguez, G. R. Eastham, D. J. Cole-Hamilton, Inorg. Chem. Commun. 2005, 8, 878–881.