PhPAd-DalPhos: Ligand-Enabled, Nickel-Catalyzed Cross-Coupling of (Hetero)aryl Electrophiles with Bulky Primary Alkylamines
Joseph P. Tassone
Department of Chemistry, Dalhousie University, Halifax, Nova Scotia, B3H 4R2 Canada
Search for more papers by this authorEmma V. England
Department of Chemistry, Dalhousie University, Halifax, Nova Scotia, B3H 4R2 Canada
Search for more papers by this authorPreston M. MacQueen
Department of Chemistry, Dalhousie University, Halifax, Nova Scotia, B3H 4R2 Canada
Search for more papers by this authorDr. Michael J. Ferguson
X-ray Crystallography Laboratory, Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2 Canada
Search for more papers by this authorCorresponding Author
Prof. Dr. Mark Stradiotto
Department of Chemistry, Dalhousie University, Halifax, Nova Scotia, B3H 4R2 Canada
Search for more papers by this authorJoseph P. Tassone
Department of Chemistry, Dalhousie University, Halifax, Nova Scotia, B3H 4R2 Canada
Search for more papers by this authorEmma V. England
Department of Chemistry, Dalhousie University, Halifax, Nova Scotia, B3H 4R2 Canada
Search for more papers by this authorPreston M. MacQueen
Department of Chemistry, Dalhousie University, Halifax, Nova Scotia, B3H 4R2 Canada
Search for more papers by this authorDr. Michael J. Ferguson
X-ray Crystallography Laboratory, Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2 Canada
Search for more papers by this authorCorresponding Author
Prof. Dr. Mark Stradiotto
Department of Chemistry, Dalhousie University, Halifax, Nova Scotia, B3H 4R2 Canada
Search for more papers by this authorGraphical Abstract
Abstract
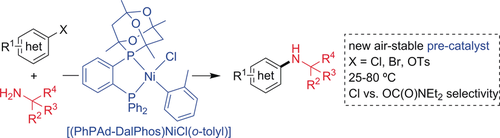
The base metal-catalyzed C−N cross-coupling of bulky α,α,α-trisubstituted primary alkylamines with (hetero)aryl electrophiles represents a challenging and under-developed class of transformations that is of significant potential utility, including in the synthesis of lipophilic active pharmaceutical ingredients. Herein, we report that a new, air-stable Ni(II) pre-catalyst incorporating the optimized ancillary ligand PhPAd-DalPhos enables such transformations of (hetero)aryl chloride, bromide, and tosylate electrophiles to be carried out for the first time with substrate scope rivalling that achieved using state-of-the-art Pd catalysts, including room temperature cross-couplings of (hetero)aryl chlorides that are unprecedented for any catalyst (Pd, Ni, or other).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201812862-sup-0001-misc_information.pdf2.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1P. Ruiz-Castillo, S. L. Buchwald, Chem. Rev. 2016, 116, 12564.
- 2J. D. Hayler, D. K. Leahy, E. M. Simmons, Organometallics 2019, 38, 36.
- 3
- 3aV. V. Grushin, H. Alper, Chem. Rev. 1994, 94, 1047;
- 3bS. Z. Tasker, E. A. Standley, T. F. Jamison, Nature 2014, 509, 299.
- 4M. Marín, R. J. Rama, M. C. Nicasio, Chem. Rec. 2016, 16, 1819.
- 5C. M. Lavoie, M. Stradiotto, ACS Catal. 2018, 8, 7228.
- 6N. H. Park, G. Teverovskiy, S. L. Buchwald, Org. Lett. 2014, 16, 220.
- 7S. Z. Ge, R. A. Green, J. F. Hartwig, J. Am. Chem. Soc. 2014, 136, 1617.
- 8V. Ritleng, M. Henrion, M. J. Chetcuti, ACS Catal. 2016, 6, 890.
- 9J. F. Hartwig, Acc. Chem. Res. 2008, 41, 1534.
- 10D. S. Surry, S. L. Buchwald, Angew. Chem. Int. Ed. 2008, 47, 6338; Angew. Chem. 2008, 120, 6438.
- 11C. M. Lavoie, P. M. MacQueen, N. L. Rotta-Loria, R. S. Sawatzky, A. Borzenko, A. J. Chisholm, B. K. V. Hargreaves, R. McDonald, M. J. Ferguson, M. Stradiotto, Nat. Commun. 2016, 7, 11073.
- 12C. M. Lavoie, R. McDonald, E. R. Johnson, M. Stradiotto, Adv. Synth. Catal. 2017, 359, 2972.
- 13
- 13aJ. S. K. Clark, C. M. Lavoie, P. M. MacQueen, M. J. Ferguson, M. Stradiotto, Organometallics 2016, 35, 3248;
- 13bJ. P. Tassone, P. M. MacQueen, C. M. Lavoie, M. J. Ferguson, R. McDonald, M. Stradiotto, ACS Catal. 2017, 7, 6048;
- 13cJ. S. K. Clark, R. T. McGuire, C. M. Lavoie, M. J. Ferguson, M. Stradiotto, Organometallics 2019, 38, 167.
- 14C. M. Lavoie, P. M. MacQueen, M. Stradiotto, Chem. Eur. J. 2016, 22, 18752.
- 15
- 15aJ. Liu, D. Obando, V. Liao, T. Lifa, R. Codd, Eur. J. Med. Chem. 2011, 46, 1949;
- 15bL. Wanka, K. Iqbal, P. R. Schreiner, Chem. Rev. 2013, 113, 3516;
- 15cT. W. Johnson, R. A. Gallego, M. P. Edwards, J. Med. Chem. 2018, 61, 6401.
- 16For select examples of other metal-mediated methods to form C−N bonds using bulky, primary amines, see:
- 16aA. M. Berman, J. S. Johnson, J. Org. Chem. 2006, 71, 219;
- 16bT. J. Barker, E. R. Jarvo, Angew. Chem. Int. Ed. 2011, 50, 8325; Angew. Chem. 2011, 123, 8475;
- 16cM. Mailig, R. P. Rucker, G. Lalic, Chem. Commun. 2015, 51, 11048;
- 16dJ. Gui, C.-M. Pan, Y. Jin, T. Qin, J. C. Lo, B. J. Lee, S. H. Spergel, M. E. Mertzman, W. J. Pitts, T. E. La Cruz, M. A. Schmidt, N. Darvatkar, S. R. Natarajan, P. S. Baran, Science 2015, 348, 886.
- 17P. Ruiz-Castillo, D. G. Blackmond, S. L. Buchwald, J. Am. Chem. Soc. 2015, 137, 3085.
- 18Y. Zhang, G. Lavigne, N. Lugan, V. César, Chem. Eur. J. 2017, 23, 13792.
- 19J. Jiang, H. Zhu, Y. Shen, T. Tu, Org. Chem. Front. 2014, 1, 1172.
- 20
- 20aE. M. Wiensch, J. Montgomery, Angew. Chem. Int. Ed. 2018, 57, 11045; Angew. Chem. 2018, 130, 11211;
- 20bA. J. Nett, S. Cañellas, Y. Higuchi, M. T. Robo, J. M. Kochkodan, M. T. Haynes, J. W. Kampf, J. Montgomery, ACS Catal. 2018, 8, 6606.
- 21C. Li, Y. Kawamata, H. Nakamura, J. C. Vantourout, Z. Liu, Q. Hou, D. Bao, J. T. Starr, J. Chen, M. Yan, P. S. Baran, Angew. Chem. Int. Ed. 2017, 56, 13088; Angew. Chem. 2017, 129, 13268.
- 22T. Harada, Y. Ueda, T. Iwai, M. Sawamura, Chem. Commun. 2018, 54, 1718.
- 23T. Iwai, T. Harada, H. Shimada, K. Asano, M. Sawamura, ACS Catal. 2017, 7, 1681.
- 24N. Hazari, P. R. Melvin, M. M. Beromi, Nat. Rev. Chem. 2017, 1, 0025.
- 25J. S. K. Clark, C. N. Voth, M. J. Ferguson, M. Stradiotto, Organometallics 2017, 36, 679.
- 26Complete experimental details and characterization data are provided in the Supporting Information. CCDC 1877193 and 1877194 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 27V. Snieckus, Chem. Rev. 1990, 90, 879.
- 28
- 28aB. M. Rosen, K. W. Quasdorf, D. A. Wilson, N. Zhang, A.-M. Resmerita, N. K. Garg, V. Percec, Chem. Rev. 2011, 111, 1346;
- 28bB.-J. Li, D.-G. Yu, C.-L. Sun, Z.-J. Shi, Chem. Eur. J. 2011, 17, 1728;
- 28cM. Tobisu, N. Chatani, Acc. Chem. Res. 2015, 48, 1717;
- 28dJ. Schranck, P. Furer, V. Hartmann, A. Tlili, Eur. J. Org. Chem. 2017, 3496;
- 28eP. M. MacQueen, M. Stradiotto, Synlett 2017, 28, 1652.
- 29P. M. MacQueen, J. P. Tassone, C. Diaz, M. Stradiotto, J. Am. Chem. Soc. 2018, 140, 5023.