Control Mechanism for cis Double-Bond Formation by Polyunsaturated Fatty-Acid Synthases
Graphical Abstract
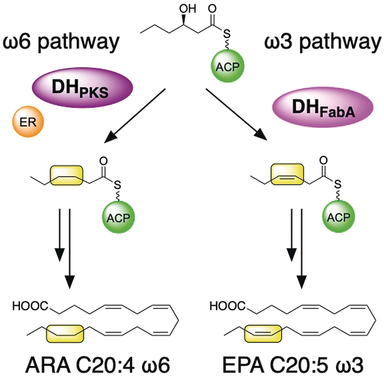
Growing chains: Eicosapentaenoic acid (EPA) synthase and arachidonic acid (ARA) synthase, both of which are composed of large enzyme complexes with multiple catalytic domains, utilize two types of dehydratase (DH) domains. The domain used depends on the carbon-chain length and can lead to introduction of either saturation or cis double bonds to the growing acyl chains. ACP=acyl-carrier protein, PKS=polyketide synthase.
Abstract
Polyunsaturated fatty acids (PUFAs) such as docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), and arachidonic acid (ARA) are essential fatty acids for humans. Some microorganisms biosynthesize these PUFAs through PUFA synthases composed of four subunits with multiple catalytic domains. These PUFA synthases each create a specific PUFA without undesirable byproducts, even though the multiple catalytic domains in each large subunit are very similar. However, the detailed biosynthetic pathways and mechanisms for controlling final-product profiles are still obscure. In this study, the FabA-type dehydratase domain (DHFabA) in the C-subunit and the polyketide synthase-type dehydratase domain (DHPKS) in the B-subunit of ARA synthase were revealed to be essential for ARA biosynthesis by in vivo gene exchange assays. Furthermore, in vitro analysis with truncated recombinant enzymes and C4- to C8-acyl ACP substrates showed that ARA and EPA synthases utilized two types of DH domains, DHPKS and DHFabA, depending on the carbon-chain length, to introduce either saturation or cis double bonds to growing acyl chains.