Ultra-High-Molecular-Weight Polymers Produced by the Immortal Phosphine-Based Catalyst System
Yun Bai
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
Search for more papers by this authorDr. Jianghua He
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Yuetao Zhang
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
Search for more papers by this authorYun Bai
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
Search for more papers by this authorDr. Jianghua He
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Yuetao Zhang
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
Search for more papers by this authorGraphical Abstract
Abstract
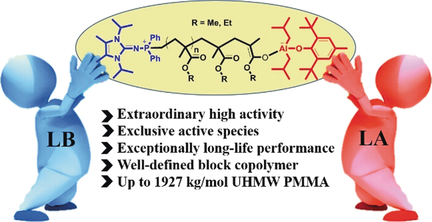
A strong organophosphorus superbase, N-(diphenylphosphanyl)-1,3-diisopropyl-4,5-dimethyl-1,3-dihydro-2H-imidazol-2-imine (IAP3) was combined with a sterically encumbered but modestly acidic Lewis acid (LA), (4-Me-2,6-tBu2-C6H2O)AliBu2 ((BHT)AliBu2), to synergistically promote the frustrated Lewis pair (FLP)-catalyzed living polymerization of methyl methacrylate (MMA), achieving ultrahigh molecular weight (UHMW) poly(methyl methacrylate) (PMMA) with Mn up to 1927 kg mol−1 and narrow molecular weight distribution (MWD) at room temperature (RT). This FLP catalyst system exhibits exceptionally long lifetime polymerization performance even in the absence of free MMA, which could reinitiate the desired living polymerization after the resulting system was held at RT for 24 h.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201811946-sup-0001-misc_information.pdf2.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aN. Hadjichristidis, A. Hirao, Anionic Polymerization: Principles, Practice, Strength, Consequences and Applications, Springer, Heidelberg, 2015;
10.1007/978-4-431-54186-8 Google Scholar
- 1bD. Baskaran, Prog. Polym. Sci. 2003, 28, 521–581.
- 2
- 2a“Cationic Polymerization of Heterocyclics”: Cationic Polymerizations (Eds.: ), CRC, Boca Raton, 1996, pp. 451–568;
10.1201/9780585400433-8 Google Scholar
- 2bA. Kanazawa, S. Kanaoka, S. Aoshima, Chem. Lett. 2010, 39, 1232–1237;
- 2cY. Kwon, R. Faust, Adv. Polym. Sci. 2004, 167, 107–136.
- 3
- 3aO. W. Webster, W. R. Hertler, D. Y. Sogah, W. B. Farnham, T. V. Rajanbabu, J. Am. Chem. Soc. 1983, 105, 5706–5708;
- 3bO. W. Webster, J. Polym. Sci. Part A 2000, 38, 2855–2860;
- 3cM. R. Kalourkoti, O. W. Webster, C. S. Patrikios, Group Transfer Polymerization Encyclopedia of Polymer Science and Technology, Vol. 99, Wiley, Hoboken, 2013, pp. 1–17. DOI: 10.1002/0471440264.pst603.
- 4
- 4a Controlled/Living Radical Polymerization: Progress in ATRP (Eds.: ), ACS Symposium Series, American Chemical Society, Washington, DC, 2009;
- 4bA. Anastasaki, V. Nikolaou, G. Nurumbetov, P. Wilson, K. Kempe, J. F. Quinn, T. P. Davis, M. R. Whittaker, D. M. Haddleton, Chem. Rev. 2016, 116, 835–877;
- 4cM. Ouchi, M. Sawamoto, Macromolecules 2017, 50, 2603–2614.
- 5
- 5aV. Percec, T. Guliashvili, J. S. Ladislaw, A. Wistrand, A. Stjerndahl, M. J. Sienkowska, M. J. Monteiro, S. Sahoo, J. Am. Chem. Soc. 2006, 128, 14156–14165;
- 5bB. Mao, L. Gan, Y. Gan, Polymer 2006, 47, 3017–3020;
- 5cR. W. Simms, M. F. Cunningham, Macromolecules 2007, 40, 860–866;
- 5dL. Mueller, W. Jakubowski, K. Matyjaszewski, J. Pietraskik, P. Kwiatkowski, W. Chaladaj, J. Jurczak, Eur. Polym. J. 2011, 47, 730–734;
- 5eE. Read, A. Guinaudeau, D. J. Wilson, A. Cadix, F. Violleau, M. Destarac, Polym. Chem. 2014, 5, 2202–2207;
- 5fN. P. Truong, M. V. Dussert, M. R. Whittaker, J. F. Quinn, T. P. Davis, Polym. Chem. 2015, 6, 3865–3874;
- 5gZ. Huang, J. Chen, L. Z. Zhang, Z. Cheng, X. Zhu, Polymer 2016, 8, 59;
- 5hZ. Liu, Y. Lv, Z. An, Angew. Chem. Int. Ed. 2017, 56, 13852–13856; Angew. Chem. 2017, 129, 14040–14044;
- 5iR. N. Carmean, T. E. Becker, M. B. Sims, B. S. Sumerlin, Chem 2017, 2, 93–101.
- 6J. Rzayev, J. Penelle, Angew. Chem. Int. Ed. 2004, 43, 1691–1694; Angew. Chem. 2004, 116, 1723–1726.
- 7T. Arita, Y. Kayama, K. Ohno, Y. Tsujii, T. Fukuda, Polymer 2008, 49, 2426–2429.
- 8S. K. Varshney, J. P. Hautekeer, R. Fayt, R. Jérôme, Ph. Teyssié, Macromolecules 1990, 23, 2618–2622.
- 9J. P. Hautekeer, S. K. Varshney, R. Fayt, C. Jacobs, R. Jérôme, P. Teyssié, Macromolecules 1990, 23, 3893–3898.
- 10
- 10aE. Y.-X. Chen, Top. Curr. Chem. 2013, 334, 239–260;
- 10bE. Piedra-Arroni, A. Amgoune, D. Bourissou, Dalton Trans. 2013, 42, 9024–9029;
- 10cY. Zhang, G. M. Miyake, E. Y.-X. Chen, Angew. Chem. Int. Ed. 2010, 49, 10158–10162; Angew. Chem. 2010, 122, 10356–10360;
- 10dY. Zhang, G. M. Miyake, M. G. John, L. Falivene, L. Caporaso, L. Cavallo, E. Y.-X. Chen, Dalton Trans. 2012, 41, 9119–9134;
- 10eT. Xu, E. Y.-X. Chen, J. Am. Chem. Soc. 2014, 136, 1774–1777;
- 10fJ. He, Y. Zhang, E. Y.-X. Chen, Synlett 2014, 25, 1534–1538;
- 10gY. Jia, W. Ren, S. Liu, T. Xu, Y. Wang, X. Lu, ACS Macro Lett. 2014, 3, 896–899;
- 10hJ. Chen, E. Y.-X. Chen, Isr. J. Chem. 2015, 55, 216–225;
- 10iP. Xu, X. Xu, ACS Catal. 2018, 8, 198–202;
- 10jE. Piedra-Arroni, C. Ladaviere, A. Amgoune, D. Bourissou, J. Am. Chem. Soc. 2013, 135, 13306–13309;
- 10kJ. Zhu, E. Y.-X. Chen, J. Am. Chem. Soc. 2015, 137, 12506–12509;
- 10lX. Li, B. Wang, H. Ji, Y. Li, Catal. Sci. Technol. 2016, 6, 7763–7772;
- 10mQ. Wang, W. Zhao, J. He, Y. Zhang, E. Y.-X. Chen, Macromolecules 2017, 50, 123–136;
- 10nH. Zhang, Y. Nie, X. Zhi, H. Du, J. Yang, Chem. Commun. 2017, 53, 5155–5158;
- 10oH. Ji, B. Wang, L. Pan, Y. Li, Green Chem. 2018, 20, 641–648;
- 10pJ. Yang, H. Wu, Y. Li, X. Zhang, D. Darensbourg, Angew. Chem. Int. Ed. 2017, 56, 5774–5779; Angew. Chem. 2017, 129, 5868–5873;
- 10qC. Zhang, H. Wu, Y. Li, J. Yang, X. Zhang, Nat. Commun. 2018, 9, 2137;
- 10rM. G. Knaus, M. M. Giuman, A. Pöthig, B. Rieger, J. Am. Chem. Soc. 2016, 138, 7776–7781;
- 10sW. Nzahou Ottou, E. Conde-Mendizabal, A. Pascual, A.-L. Wirotius, D. Bourichon, J. Vignolle, F. Robert, Y. Landais, J.-M. Sotiropoulos, K. Miqueu, Macromolecules 2017, 50, 762–774;
- 10tP. Xu, Y. Yao, X. Xu, Chem. Eur. J. 2017, 23, 1263–1267;
- 10uM. Wang, F. Nudelman, R. R. Matthes, M. P. Shaver, J. Am. Chem. Soc. 2017, 139, 14232–14236;
- 10vY. Hosoi, A. Takasu, S. Matsuoka, M. Hayashi, J. Am. Chem. Soc. 2017, 139, 15005–15012.
- 11Selected some reviews and recent examples on activation of small molecules by FLPs:
- 11aD. W. Stephan, Science 2016, 354, aaf 7229;
- 11bD. W. Stephan, G. Erker, Angew. Chem. Int. Ed. 2015, 54, 6400–6441; Angew. Chem. 2015, 127, 6498–6541;
- 11cD. W. Stephan, Acc. Chem. Res. 2015, 48, 306–316;
- 11dD. W. Stephan, J. Am. Chem. Soc. 2015, 137, 10018–10032;
- 11eW. Meng, X. Feng, H. Du, Acc. Chem. Res. 2018, 51, 191–201;
- 11fC. Tang, Q. Liang, A. R. Jupp, T. C. Johnstone, R. C. Neu, D. Song, S. Grimme, D. W. Stephan, Angew. Chem. Int. Ed. 2017, 56, 16588–16592; Angew. Chem. 2017, 129, 16815–16819;
- 11gA. Simonneau, R. Turrel, L. Vendier, M. Etienne, Angew. Chem. Int. Ed. 2017, 56, 12268–12272; Angew. Chem. 2017, 129, 12436–12440;
- 11hL. Liu, L. Cao, Y. Shao, D. W. Stephan, J. Am. Chem. Soc. 2017, 139, 10062–10071;
- 11iZ. Jian, G. Kehr, C. G. Daniliuc, B. Wibbeling, T. Wiegand, M. Siedow, H. Eckert, M. Bursch, S. Grimme, G. Erker, J. Am. Chem. Soc. 2017, 139, 6474–6483;
- 11jM. Trunk, J. F. Teichert, A. Thomas, J. Am. Chem. Soc. 2017, 139, 3615–3618;
- 11kZ. Mo, T. Szilvasi, Y. Zhou, S. Yao, M. Driess, Angew. Chem. Int. Ed. 2017, 56, 3699–3702; Angew. Chem. 2017, 129, 3753–3756;
- 11lO. J. Metters, S. J. K. Forrest, H. A. Sparkes, I. Manners, D. F. Wass, J. Am. Chem. Soc. 2016, 138, 1994–2003.
- 12J. He, Y. Zhang, L. Falivene, L. Caporaso, L. Cavallo, E. Y.-X. Chen, Macromolecules 2014, 47, 7765–7774.
- 13Y. Jia, Y. Wang, W. Ren, T. Xu, J. Wang, X. Lu, Macromolecules 2014, 47, 1966–1972.
- 14Q. Wang, W. Zhao, S. Zhang, J. He, Y. Zhang, E. Y.-X. Chen, ACS Catal. 2018, 8, 3571–3578.
- 15M. A. Wünsche, P. Mehlmann, T. Witteler, F. Buß, P. Rathmann, F. Dielmann, Angew. Chem. Int. Ed. 2015, 54, 11857–11860; Angew. Chem. 2015, 127, 12024–12027.
- 16F. Buß, P. Mehlmann, C. Mück-Lichtenfeld, K. Bergander, F. Dielmann, J. Am. Chem. Soc. 2016, 138, 1840–1843.
- 17P. Mehlmann, C. Mück-Lichtenfeld, T. T. Y. Tan, F. Dielmann, Chem. Eur. J. 2017, 23, 5929–5933.
- 18F. Buß, P. Rotering, C. Mück-Lichtenfeld, F. Dielmann, Dalton Trans. 2018, 47, 10420–10424.
- 19F. Buß, C. Mück-Lichtenfeld, P. Mehlmann, F. Dielmann, Angew. Chem. Int. Ed. 2018, 57, 4951–4955; Angew. Chem. 2018, 130, 5045–5049.
- 20
- 20aM. A. Beckett, D. S. Brassinton, S. J. Coles, M. B. Hursthouse, Inorg. Chem. Commun. 2000, 3, 530–533;
- 20bZ. M. Heiden, A. P. Lathem, Organometallics 2015, 34, 1818–1827.