Metal-Free Radical Borylation of Alkyl and Aryl Iodides
Graphical Abstract
Abstract
A metal-free radical borylation of alkyl and aryl iodides with bis(catecholato)diboron (B2cat2) as the boron source under mild conditions is introduced. The borylation reaction is operationally easy to conduct and shows high functional group tolerance and broad substrate scope. Radical clock experiments and density functional theory calculations provide insights into the mechanism and rate constants for C-radical borylation with B2cat2 are disclosed.
Boronic esters are highly valuable synthetic precursors, since the boronic ester moiety can be transformed into a wide range of useful functional groups.1 Therefore, methods that allow to efficiently prepare alkyl and aryl boronates are demanded.
In recent years, various processes for transition metal catalyzed borylation of alkyl halides with diborons to access alkyl boronate esters have been developed (Scheme 1 A).2, 3, 4, 5, 6 Steel, Marder, and Liu disclosed a CuCl/Xantphos-catalyzed borylation of alkyl halides.2 The Ito group reported a similar reaction with diboron using a CuCl/Xantphos catalyst and stoichiometric KOtBu.3 Fu reported borylation of various alkyl electrophiles with a NiBr2/pybox catalyst,4 and Marder introduced a ZnCl2/N-heterocyclic carbene catalyst system for the borylation of unactivated alkyl halides.5 Recently, Cook disclosed a MnBr2/tetramethylethylenediamine-catalyzed borylation of alkyl halides using EtMgBr as a stoichiometric activator.6 However, all these valuable methods require a transition metal catalyst, a ligand and a stoichiometric amount of a base. To our knowledge, metal and additive-free radical borylation of alkyl halides with diborons is unknown.7
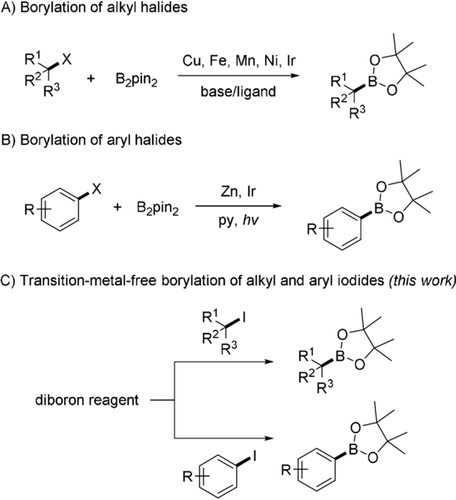
Borylation of alkyl and aryl halides with diboron reagents [B2pin2=bis(pinacolato)diboron].
Arylboronates can be obtained from haloarenes and diborons via reactive aryl radicals8, 9, 10-12, 13 (Scheme 1 B). Along these lines, Marder developed a zinc(II)-catalyzed borylation of aryl halides using a stoichiometric base8 and photoinduced aryl halide borylation was reported by Larionov,9 Li10 and Fu.11 Recently, a pyridine-catalyzed radical borylation of aryl halides was disclosed by Jiao.12 We present herein mild radical borylation of both alkyl and aryl iodides with commercial bis(catecholato)diboron as the boron source (Scheme 1 C).
We first explored the reaction of 1-iodooctane (1 a) with bis(catecholato)diboron (2 a) to alkyl boronate 3 a under blue LED irradiation at ambient temperature using different solvents (Table 1). Due to instability of n-octylcatecholborane, the crude product was transesterified with pinacol and NEt3 to give 3 a. In 1,2-dichloroethane (1,2-DCE), borylation did not proceed and 1 a was recovered (Table 1, entry 1). In acetonitrile or tetrahydrofuran (THF), trace amounts of 3 a were detected (entries 2, 3), but in methanol an encouraging 8 % yield was obtained (entry 4). Significant improvement of the yield to 71 % was achieved by using N,N-dimethylformamide (DMF)14 (entry 5). Replacing B2cat2 by bis(pinacolato)diboron (B2pin2) or bis(neopentylglycolato)diboron (B2neo2) under otherwise identical conditions did not lead to any product formation (entries 6, 7). Using tetrahydroxydiboron [B2(OH)4] as the trapping reagent and subsequent treatment with pinacol and magnesium sulfate, only 7 % of 3 a was obtained (entry 8). Increasing the amount of B2cat2 to 4 equivalents led to a further improvement (88 %, entry 9). A control experiment in the absence of light irradiation proceeded in low yield (entry 10).

Entry |
n |
Solvent |
Yield [%] |
|---|---|---|---|
1 |
2 |
1,2-DCE |
0 |
2 |
2 |
MeCN |
trace |
3 |
2 |
THF |
trace |
4 |
2 |
MeOH |
8 |
5 |
2 |
DMF |
71 |
6[b] |
2 |
DMF |
0 |
7[c] |
2 |
DMF |
0 |
8[d] |
2 |
DMF |
7 |
9 |
4 |
DMF |
88 |
10[e] |
4 |
DMF |
15 |
- [a] Reaction conditions: 1 a (0.2 mmol), 2 a (n×0.2 mmol), solvent (0.6 mL), 10 W blue LED, Ar, RT, 24 h; pinacol (0.8 mmol), NEt3 (0.7 mL), 1 h, isolated yields. [b] B2pin2 was used. [c] B2neo2 was used. [d] B2(OH)4 was used. [e] In the dark.
Under optimized conditions, substrate scope was investigated. Borylation of 3-methylbutyliodide (1 b) to 3 b proceeded in 80 % yield (Scheme 2). With 1-chloro-6-iodohexane (1 c) containing both an iodine and a chlorine atom, the borylation occurred chemoselectively by replacing the I-atom (3 c, 94 %). The primary alkyl iodide 1 d bearing a perfluoroalkyl chain worked well (see 3 d) and an alkyl iodide containing a carbazole moiety participated to provide 3 e (70 %). (2-Iodoethyl)benzene (1 f) and 4-(iodomethyl)cyclohex-1-ene (1 g) were also borylated in good yields (91 % and 82 %). Alkynyl- (1 h), nitrile- (1 i), ester- (1 j), ketone- (1 k), and aldehyde-functionalities (1 l) are tolerated and the products 3 h–l were isolated in good yields (56–80 %). Borodeiodination of secondary alkyl iodides worked well, as shown for 2-iodoheptane (1 m) and 6-iodo-2-methylhept-2-ene (1 n) to provide 3 m and 3 n in 91 % and 89 % yield. Iodides containing cyclohexane, tetrahydropyran, Boc-protected piperidine (Boc=tert-butoxycarbonyl), arene and phenyl alkyl ether (1 o–s) turned out to be good substrates for the borylation. Compound 1 t with both naphthyl and perfluorobutyl groups performed well to give 3 t in 86 % yield. 1-Iodoadamantane (1 u) was smoothly converted to 3 u (90 %), but tert-butyliodide could not be borylated under the standard conditions.
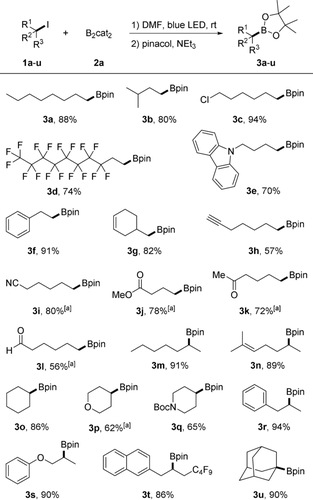
Scope of alkyl iodides. Reaction conditions: 1 a–u (0.2 mmol), 2 a (0.8 mmol), DMF (0.6 mL), 10 W blue LED, Ar, RT, 24 h; pinacol (0.8 mmol), NEt3 (0.7 mL), 1 h, isolated yields. [a] 2 equiv of 2 a was used.
We were very pleased to find that under the same conditions aryl iodides can also be borylated (Scheme 3). However, as compared to the alkyl iodides slightly lower yields are generally achieved. Iodobenzene (4 a) afforded phenyl pinacolboronate (5 a, 61 %) and iodoarenes 4 b–g bearing para-substituents performed well to give the aryl boronates 4 b–g in 46–71 % yield. Meta and ortho-substituted aryl iodides 4 h and 4 i provided 5 h and 5 i in good yields (73 % each). Borylation of 1-iodonaphthalene (4 j), 9-iodophenanthrene (4 k) and 3-iodothiophene (4 l) was also successful (5 j–l). Our method, albeit less efficiently, is also applicable to the preparation of vinyl boronates, as documented by the borylation of 1-iodocyclohex-1-ene (5 m, 32 %).
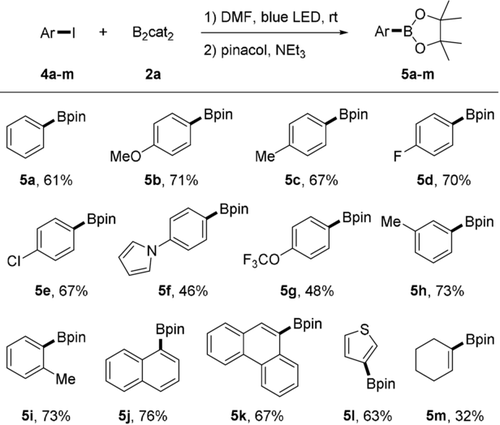
Scope of aryl iodides. Reaction conditions: 4 a-m (0.2 mmol), 2 a (0.8 mmol), DMF (0.6 mL), 10 W blue LED, Ar, RT, 24 h; pinacol (0.8 mmol), NEt3 (0.7 mL), 1 h, isolated yields.
The facts that light was required and that conditions are similar to those reported by us and Aggarwal for alkyl radical borylations7a,7d, 14a indicated that these transformations are likely radical in nature. We therefore carried out radical clock experiments (Scheme 4). Cyclopropylmethyl iodide (6) gave exclusively the ring opening product 7, but 6-Iodo-1-hexene (8) reacted mainly to the uncyclized boronate 9 a. Still, formation of small amounts of the cyclized product 9 b indicated that radicals are involved. Moreover, this experiment revealed that trapping of a primary C-radical with B2cat2 to be very fast. Successful cyclizing borylation of aryl iodide 10 to 11 b further supported the radical mechanism. Such cyclizing borylations cannot be achieved using ionic type borylation processes.
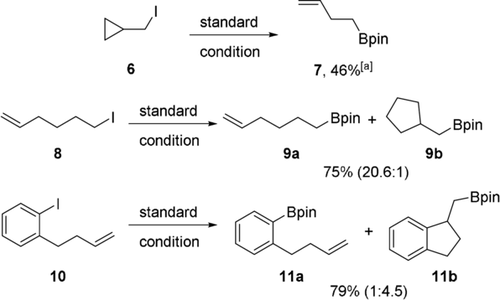
Mechanistic experiments, isolated yields. [a] Yield was determined by 1H NMR spectroscopy with 1,1,2,2-tetrachloroethane as the internal standard.
The radical clock experiment with iodide 8 was chosen to estimate the rate constant for trapping of a primary C-radical with B2cat2. To this end, a series of reactions with 6-iodo-1-hexene (8) were conducted varying the concentration of diboron 2 a. Using the ratio of non-cyclized 9 a to cyclized product 9 b at a given diboron concentration and considering the known rate constant (2.3×105 s−1 at 25 °C15) for the 5-exo-cyclization of the 5-hexenyl radical, the second order rate constant for the borylation of the primary C-radical derived from 8 with B2cat2 was estimated to be 3.8×106 m−1 s−1 (for details, see the Supporting Information (SI)). In analogy, we estimated the rate constant for trapping of the aryl radical derived from 1-(3-butenyl)-2-iodo-benzene (10) with B2cat2 to lie at 7.4×107 m−1 s−1. Hence, alkyl radical trapping with B2cat2 is a very fast process and its rate constant lies in the region of that for the reduction of a primary alkyl radical with Bu3SnH.15 However, the rate constant for the reaction of B2cat2 with the aryl radical derived from 10 is around one order of magnitude smaller than that of the tin hydride reduction of a phenyl radical.16 Likely, steric effects exerted by the ortho-substituent and the bulky diborane play a role. Notably, kinetic data on radical borylation are currently unavailable in the literature.
Based on these experiments and previous results,14a a plausible mechanism is depicted in Scheme 5. Initiation proceeds via light mediated C−I bond homolysis of iodide 1 to generate the alkyl/aryl radical A which adds to B2cat2 to give the radical B. We found that for radicals B derived from secondary alkyl radicals, B−B bond homolysis is higher in energy than degenerate B−C bond homolysis to give B2cat2 and the starting radical A.14a The unusual large solvent effect can readily be understood involving the solvent. Hence, the radical B is trapped by DMF in an exothermic process to give C, best described by a weak B−B one electron σ-bond,14 which readily homolyzes to give product boronate 3 along with the DMF-complexed boryl radical D/E. The chain is closed by an I-atom transfer reaction17 of the iodide 1 via F to eventually give G and radical A.
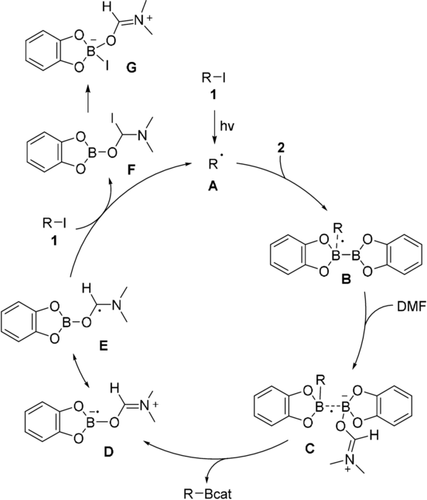
Proposed mechanism.
The I-atom transfer was further investigated using DFT calculations (see SI for details). We looked at the spin density plots of the DMF-complexed boryl radical D and found highest spin density at the C-atom and only little spin at the boron atom (Figure 1). Therefore, the complexed boryl radical D is best described by resonance structure E, which certainly also influences its reactivity. In fact, I-transfer from CH3I to the C-atom in E to give F proceeds via a low barrier (9.5 kcal mol−1), whereas the barrier for I-transfer to the B-atom leading directly to G occurs at significantly higher barrier (18.3 kcal mol−1) (Figure 2). In a polar solvent, however, iodide F is not stable and easily isomerizes (ΔG=−8 kcal mol−1) to the zwitterion G. The barrier in vacuum is only 3.7 kcal mol−1, but becomes negative when solvation energies are included. Thus, F is most likely not a minimum structure under polar solvation conditions, but rather an ion pair (E+/I−) which is formally formed by heterolytic fission of the C−I bond in F or by charge transfer from E to the I-atom. Zwitterion G is immediately formed by collapse of this ion pair.
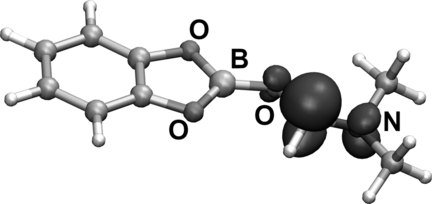
Calculated spin density of radical D/E (PBE0-D3/def2-TZVP, isosurface value: 0.01 a.u.).
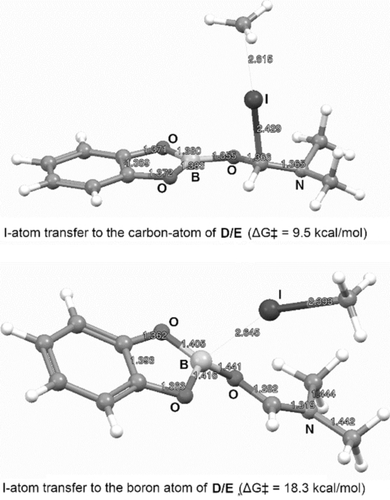
Transition structures for I-atom transfer from CH3I to radical D/E (PBE0-D3/def2-TZVP).
In summary, a method for metal and additive-free borylation of alkyl and aryl iodides was introduced. The reaction proceeds under very mild conditions in good to excellent yields with a broad range of alkyl and aryl iodides. As compared to the reactions with aryl iodides, alkyl iodides generally lead to higher yields. Radical clock experiments and DFT calculations support the suggested radical chain mechanism.
Acknowledgements
We thank the European Research Council (ERC Advanced Grant agreement No. 692640) for financial support.
Conflict of interest
The authors declare no conflict of interest.





