Catalytic Enantioselective Aldol Reactions of Unprotected Carboxylic Acids under Phosphine Oxide Catalysis
Corresponding Author
Dr. Shunsuke Kotani
Graduate School of Pharmaceutical Sciences, Kumamoto University, 5-1 Oe-honmachi, Chuo-ku, Kumamoto, 862-0973 Japan
Priority Organization for Innovation and Excellence, Kumamoto University, 5-1 Oe-honmachi, Chuo-ku, Kumamoto, 862-0973 Japan
Search for more papers by this authorYusaku Yoshiwara
Graduate School of Pharmaceutical Sciences, Kumamoto University, 5-1 Oe-honmachi, Chuo-ku, Kumamoto, 862-0973 Japan
Search for more papers by this authorDr. Masamichi Ogasawara
Graduate School of Science and Technology, Tokushima University, 2-1 Minamijyousanjima-cho, Tokushima, 770-8506 Japan
Search for more papers by this authorDr. Masaharu Sugiura
Faculty of Pharmaceutical Sciences, Sojo University, 4-22-1 Ikeda, Nishi-ku, Kumamoto, 860-0082 Japan
Search for more papers by this authorCorresponding Author
Dr. Makoto Nakajima
Graduate School of Pharmaceutical Sciences, Kumamoto University, 5-1 Oe-honmachi, Chuo-ku, Kumamoto, 862-0973 Japan
Search for more papers by this authorCorresponding Author
Dr. Shunsuke Kotani
Graduate School of Pharmaceutical Sciences, Kumamoto University, 5-1 Oe-honmachi, Chuo-ku, Kumamoto, 862-0973 Japan
Priority Organization for Innovation and Excellence, Kumamoto University, 5-1 Oe-honmachi, Chuo-ku, Kumamoto, 862-0973 Japan
Search for more papers by this authorYusaku Yoshiwara
Graduate School of Pharmaceutical Sciences, Kumamoto University, 5-1 Oe-honmachi, Chuo-ku, Kumamoto, 862-0973 Japan
Search for more papers by this authorDr. Masamichi Ogasawara
Graduate School of Science and Technology, Tokushima University, 2-1 Minamijyousanjima-cho, Tokushima, 770-8506 Japan
Search for more papers by this authorDr. Masaharu Sugiura
Faculty of Pharmaceutical Sciences, Sojo University, 4-22-1 Ikeda, Nishi-ku, Kumamoto, 860-0082 Japan
Search for more papers by this authorCorresponding Author
Dr. Makoto Nakajima
Graduate School of Pharmaceutical Sciences, Kumamoto University, 5-1 Oe-honmachi, Chuo-ku, Kumamoto, 862-0973 Japan
Search for more papers by this authorGraphical Abstract
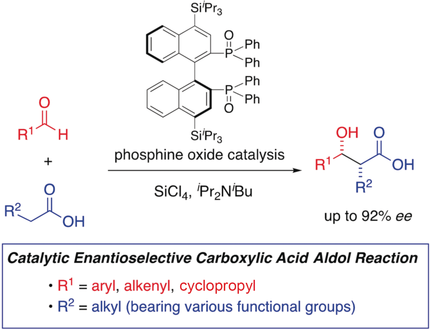
Various unprotected carboxylic acids undergo enantioselective aldol reactions in the presence of a chiral phosphine oxide as a Lewis base catalyst. The carboxylic acids were activated with silicon tetrachloride to form the bis(trichlorosilyl)enediolates in situ, which subsequently underwent an aldol reaction with an aldehyde or a ketone to produce β-hydroxycarboxylic acids in high enantioselectivities of up to 92 % ee.
Abstract
The first catalytic enantioselective aldol reaction of various unprotected carboxylic acids is described. In the presence of a chiral bis(phosphine oxide) as a Lewis base catalyst, carboxylic acids were activated with silicon tetrachloride to form the corresponding bis(trichlorosilyl)enediolates in situ, which subsequently underwent an aldol reaction with an aldehyde or a ketone to produce β-hydroxycarboxylic acids in high enantioselectivities of up to 92 % ee.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201810599-sup-0001-misc_information.pdf9.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aH. Maag in Prodrugs (Eds.: ), Springer, New York, 2007, pp. 703–729;
10.1007/978-0-387-49785-3_20 Google Scholar
- 1bC. Lamberth, J. Dinges in Bioactive Carboxylic Compound Classes: Pharmaceuticals and Agrochemicals (Eds.: ), Wiley-VCH, Weinheim, 2016, pp. 1–11.
- 2
- 2aT. Mukaiyama, J. Matsuo in Modern Aldol Reactions, Vol. 1 (Ed.: ), Wiley-VCH, Weinheim, 2004, pp. 127–160;
10.1002/9783527619566.ch3 Google Scholar
- 2bR. Mahrwald in Aldol Reactions (Ed.: ), Springer, Dordrecht, Heidelberg, 2009, pp. 69–71.
10.1007/978-1-4020-8701-1_7 Google Scholar
- 3
- 3aB. Blagoev, D. Ivanov, Synthesis 1970, 615–627;
- 3bP. Galatsis, J. J. Manwell, J. M. Blackwell, Can. J. Chem. 1994, 72, 1656–1659;
- 3cM. Parra, E. Sotoca, S. Gil, Eur. J. Org. Chem. 2003, 1386–1388.
- 4J. Mulzer, P. Lasalle, A. Chucholowski, U. Blaschek, G. Brüntrup, Tetrahedron 1984, 40, 2211–2218.
- 5K. Yu, P. Lu, J. J. Jackson, T.-A. D. Nguyen, J. Alvarado, C. E. Stivala, Y. Ma, K. A. Mack, T. W. Hayton, D. B. Collum, A. Zakarian, J. Am. Chem. Soc. 2017, 139, 527–533.
- 6F. Fringuelli, O. Piermatti, F. Pizzo, J. Org. Chem. 1995, 60, 7006–7009.
- 7
- 7aD. A. Evans, J. V. Nelson, E. Vogel, T. R. Taber, J. Am. Chem. Soc. 1981, 103, 3099–3111;
- 7bH. C. Brown, R. K. Dhar, K. Ganesan, B. Singaram, J. Org. Chem. 1992, 57, 499–504;
- 7cP. V. Ramachandran, P. B. Chanda, B. Otoo, Tetrahedron Lett. 2014, 55, 1289–1291;
- 7dP. V. Ramachandran, B. Otoo, P. B. Chanda, Tetrahedron Lett. 2015, 56, 3019–3022.
- 8C. W. Downey, M. W. Johnson, D. H. Lawrence, A. S. Fleisher, K. J. Tracy, J. Org. Chem. 2010, 75, 5351–5354.
- 9
- 9aH. Nagai, Y. Morita, Y. Shimizu, M. Kanai, Org. Lett. 2016, 18, 2276–2279;
- 9bK. Ishizawa, H. Nagai, Y. Shimizu, M. Kanai, Chem. Pharm. Bull. 2018, 66, 231–234.
- 10Y. Morita, T. Yamamoto, H. Nagai, Y. Shimizu, M. Kanai, J. Am. Chem. Soc. 2015, 137, 7075–7078.
- 11For reviews on Lewis base catalysis, see:
- 11aY. Orito, M. Nakajima, Synthesis 2006, 1391–1401;
- 11bS. E. Denmark, G. L. Beutner, Angew. Chem. Int. Ed. 2008, 47, 1560–1638; Angew. Chem. 2008, 120, 1584–1663.
- 12
- 12aM. Benaglia, S. Rossi, Org. Biomol. Chem. 2010, 8, 3824–3830;
- 12bS. Kotani, M. Sugiura, M. Nakajima, Chem. Rec. 2013, 13, 362–370.
- 13For leading papers on phosphine oxide catalyzed asymmetric cross-aldol reactions, see:
- 13aS. Kotani, Y. Shimoda, M. Sugiura, M. Nakajima, Tetrahedron Lett. 2009, 50, 4602–4605;
- 13bM. Bonsignore, M. Benaglia, F. Cozzi, A. Genoni, S. Rossi, L. Raimondi, Tetrahedron 2012, 68, 8251–8255.
- 14The stereochemistry of the carboxylic acids was determined after conversion into methyl esters 11.
- 15The enantiomeric excess of the anti isomer was low; see the Supporting Informtaion for details.
- 16S. Kotani, T. Hanamure, H. Nozaki, M. Sugiura, M. Nakajima, Tetrahedron: Asymmetry 2017, 28, 282–287.
- 17P(O)Ph3 (20 mol %) was an ineffective catalyst for the reaction.
- 18K. Mashima, K. Kusano, N. Sato, Y. Matsumura, K. Nozaki, H. Kumobayashi, N. Sayo, T. Hori, T. Ishizaki, S. Akutagawa, H. Takaya, J. Org. Chem. 1994, 59, 3064–3076.
- 19
- 19aS. Kotani, S. Aoki, M. Sugiura, M. Ogasawara, M. Nakajima, Org. Lett. 2014, 16, 4802–4805;
- 19bS. Kotani, K. Kai, Y. Shimoda, H. Hu, S. Gao, M. Sugiura, M. Ogasawara, M. Nakajima, Chem. Asian J. 2016, 11, 376–379.
- 20For facile purification and analysis, the yields and the stereoselectivities correspond to those of methyl esters 11.
- 21CCDC 1848185 ((2R,3R)-11 ba) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 22
- 22aS. E. Denmark, X. Su, Y. Nishigaichi, J. Am. Chem. Soc. 1998, 120, 12990–12991;
- 22bS. E. Denmark, S. M. Pham, R. A. Stavenger, X. Su, K.-T. Wong, Y. Nishigaichi, J. Org. Chem. 2006, 71, 3904–3922.
- 23E. J. Corey, S. S. Kim, J. Am. Chem. Soc. 1990, 112, 4976–4977.