Covalent Organic Frameworks with Chirality Enriched by Biomolecules for Efficient Chiral Separation
Sainan Zhang
State Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin, 300071 China
College of Pharmacy, Nankai University, Tianjin, 300071 China
Search for more papers by this authorYunlong Zheng
State Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin, 300071 China
College of Pharmacy, Nankai University, Tianjin, 300071 China
Search for more papers by this authorHongde An
State Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin, 300071 China
College of Pharmacy, Nankai University, Tianjin, 300071 China
Search for more papers by this authorBriana Aguila
Department of Chemistry, University of South Florida, 4202 E. Fowler Avenue, Tampa, FL, 33620 USA
Search for more papers by this authorProf. Cheng-Xiong Yang
College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorYueyue Dong
College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorProf. Wei Xie
College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorProf. Peng Cheng
College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Zhenjie Zhang
State Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin, 300071 China
College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Yao Chen
State Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin, 300071 China
College of Pharmacy, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Shengqian Ma
Department of Chemistry, University of South Florida, 4202 E. Fowler Avenue, Tampa, FL, 33620 USA
Search for more papers by this authorSainan Zhang
State Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin, 300071 China
College of Pharmacy, Nankai University, Tianjin, 300071 China
Search for more papers by this authorYunlong Zheng
State Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin, 300071 China
College of Pharmacy, Nankai University, Tianjin, 300071 China
Search for more papers by this authorHongde An
State Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin, 300071 China
College of Pharmacy, Nankai University, Tianjin, 300071 China
Search for more papers by this authorBriana Aguila
Department of Chemistry, University of South Florida, 4202 E. Fowler Avenue, Tampa, FL, 33620 USA
Search for more papers by this authorProf. Cheng-Xiong Yang
College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorYueyue Dong
College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorProf. Wei Xie
College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorProf. Peng Cheng
College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Zhenjie Zhang
State Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin, 300071 China
College of Chemistry, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Yao Chen
State Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin, 300071 China
College of Pharmacy, Nankai University, Tianjin, 300071 China
Search for more papers by this authorCorresponding Author
Prof. Shengqian Ma
Department of Chemistry, University of South Florida, 4202 E. Fowler Avenue, Tampa, FL, 33620 USA
Search for more papers by this authorGraphical Abstract
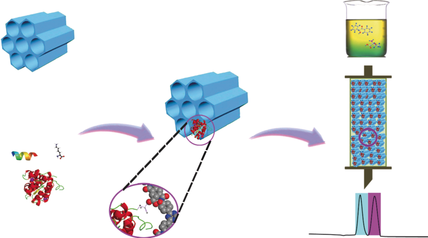
COF chirality: A general and efficient strategy has been developed to introduce chirality into covalent organic frameworks (COFs) by covalently immobilizing biomolecules into achiral COFs. The biomolecules⊂COFs can serve as chiral stationary phases for efficient chiral separation of a broad range of racemates.
Abstract
The separation of racemic compounds is important in many fields, such as pharmacology and biology. Taking advantage of the intrinsically strong chiral environment and specific interactions featured by biomolecules, here we contribute a general strategy is developed to enrich chirality into covalent organic frameworks (COFs) by covalently immobilizing a series of biomolecules (amino acids, peptides, enzymes) into achiral COFs. Inheriting the strong chirality and specific interactions from the immobilized biomolecules, the afforded biomolecules⊂COFs serve as versatile and highly efficient chiral stationary phases towards various racemates in both normal and reverse phase of high-performance liquid chromatography (HPLC). The different interactions between enzyme secondary structure and racemates were revealed by surface-enhanced Raman scattering studies, accounting for the observed chiral separation capacity of enzymes⊂COFs.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201810571-sup-0001-misc_information.pdf2.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1K. Neeraj, S. Upendra, S. Chitra, S. Bikram, Curr. Top. Med. Chem. 2012, 12, 1436–1455.
- 2S. M. Xie, L. M. Yuan, J. Sep. Sci. 2017, 40, 124–137.
- 3S. Y. Zhang, C. X. Yang, W. Shi, X. P. Yan, P. Cheng, L. Wojtas, M. J. Zaworotko, Chem 2017, 3, 281–289.
- 4S. Das, S. Xu, T. Ben, S. Qiu, Angew. Chem. Int. Ed. 2018, 57, 8629–8633; Angew. Chem. 2018, 130, 8765–8769.
- 5T. Jacobs, R. Clowes, A. I. Cooper, M. J. Hardie, Angew. Chem. Int. Ed. 2012, 51, 5192–5195; Angew. Chem. 2012, 124, 5282–5285.
- 6Q. Han, B. Qi, W. Ren, C. He, J. Niu, C. Duan, Nat. Commun. 2015, 6, 10007.
- 7J. Shen, Y. Okamato, Chem. Rev. 2016, 116, 1094–1138.
- 8P. Levkin, N. M. Maier, W. Lindner, V. Schurig, J. Chromatogr. A 2012, 1269, 270–278.
- 9A. Berthod, W. Li, D. W. Armstrong, Anal. Chem. 1992, 64, 873–879.
- 10A. Cavazzini, G. Nadalini, F. Dondi, F. Gasparrini, J. Chromatogr. A 2004, 1031, 143–158.
- 11C. Bi, X. Zheng, S. Azaria, S. Beeram, Z. Li, D. S. Hage, Separations 2016, 3, 27.
- 12Y. Song, Q. Sun, B. Aguila, S. Ma, Adv. Sci. 2018, https://doi.org/10.1002/advs.201801410.
- 13C. Qian, Q. Y. Qi, G. F. Jiang, F. Z. Cui, Y. Tian, X. Zhao, J. Am. Chem. Soc. 2017, 139, 6736–6743.
- 14N. Huang, P. Wang, D. Jiang, Nat. Rev. Mater. 2016, 1, 16068.
- 15H. Furukawa, O. M. Yaghi, J. Am. Chem. Soc. 2009, 131, 8875–8883.
- 16E. Jin, M. Asada, Q. Xu, S. Dalapati, M. A. Addicoat, M. A. Brady, H. Xu, T. Nakamura, T. Heine, Q. Chen, D. Jiang, Science 2017, 357, 673–676.
- 17H. S. Xu, S. Y. Ding, W. K. An, H. Wu, W. Wang, J. Am. Chem. Soc. 2016, 138, 11489–11492.
- 18X. Han, Q. Xia, J. Huang, Y. Liu, C. Tan, Y. Cui, J. Am. Chem. Soc. 2017, 139, 8693–8697.
- 19H. Xu, J. Gao, D. Jiang, Nat. Chem. 2015, 7, 905–912.
- 20Q. Sun, B. Aguila, L. D. Earl, C. W. Abney, L. Wojtas, P. K. Thallapally, S. Ma, Adv. Mater. 2018, 30, 1705479.
- 21Q. Fang, J. Wang, S. Gu, R. B. Kaspar, Z. Zhuang, J. Zheng, H. Guo, S. Qiu, Y. Yan, J. Am. Chem. Soc. 2015, 137, 8352–8355.
- 22Q. Sun, B. Aguila, J. Perman, T. Butts, F.-S. Xiao, S. Ma, Chem 2018, 4, 1726–1739.
- 23H. L. Qian, C. X. Yang, X. P. Yan, Nat. Commun. 2016, 7, 12104.
- 24X. Han, J. Huang, C. Yuan, Y. Liu, Y. Cui, J. Am. Chem. Soc. 2018, 140, 892–895.
- 25H. L. Qian, C. X. Yang, W. L. Wang, C. Yang, X. P. Yan, J. Chromatogr. A 2018, 1542, 1–18.
- 26Q. Sun, C. W. Fu, B. Aguila, J. Perman, S. Wang, H. Y. Huang, F. S. Xiao, S. Ma, J. Am. Chem. Soc. 2018, 140, 984–992.
- 27G. T. Hermanson, A. K. Mallia, P. K. Smith, Immobilized affinity ligand techniques, Academic Press, New York, 1992.
- 28R. Messing, Immobilized Enzymes For Industrial Reactors, Academic Press, New York, 1975.
- 29H. Weetall, Immobilized Enzyme Technology: Research and Applications, Springer Science & Business Media, Cham, 2012.
- 30C. C. F. Blake, D. F. Koenig, G. A. Mair, A. C. T. North, D. C. Phillips, V. R. Sarma, Nature 1965, 206, 757–761.
- 31Q. R. Fang, Z. Zhuang, S. Gu, R. B. Kaspar, J. Zheng, J. Wang, S. Qiu, Y. Yan, Nat. Commun. 2014, 5, 4503.
- 32B. Lu, T. C. Chung, Macromolecules 1999, 32, 8678–8680.
- 33E. T. Kang, K. L. Tan, Macromolecules 1996, 29, 6872–6879.
- 34Q. Lu, Y. Ma, H. Li, X. Guan, Y. Yusran, M. Xue, Q. Fang, Y. Yan, S. Qiu, V. Valtchev, Angew. Chem. Int. Ed. 2018, 57, 6042–6048; Angew. Chem. 2018, 130, 6150–6156.
- 35S. C. Tsang, Y. K. Chen, P. J. F. Harris, M. L. H. Green, Nature 1994, 372, 159–162.
- 36D. Samanta, A. Sarkar, Chem. Soc. Rev. 2011, 40, 2567–2592.
- 37J. S. Kahn, L. Freage, N. Enkin, M. A. A. Garcia, I. Willner, Adv. Mater. 2017, 29, 1602782.
- 38P. Li, Q. Chen, T. C. Wang, N. A. Vermeulen, B. L. Mehdi, A. Dohnalkova, N. D. Browning, D. Shen, R. Anderson, D. A. Gómez-Gualdrón, F. M. Cetin, J. Jagiello, A. M. Asiri, J. F. Stoddart, O. K. Farha, Chem 2018, 4, 1022–1034.
- 39V. Lykourinou, Y. Chen, X. S. Wang, L. Meng, T. Hoang, L. J. Ming, R. L. Musselman, S. Ma, J. Am. Chem. Soc. 2011, 133, 10382–10385.
- 40S. Yuan, J. S. Qin, L. Zou, Y. Chen, X. Wang, Q. Zhang, H. C. Zhou, J. Am. Chem. Soc. 2016, 138, 6636–6642.
- 41Y. Chen, S. Han, X. Li, Z. Zhang, S. Ma, Inorg. Chem. 2014, 53, 10006–10008.
- 42J. Gao, B. Zhao, M. E. Itkis, E. Bekyarova, H. Hu, V. Kranak, A. Yu, R. C. Haddon, J. Am. Chem. Soc. 2006, 128, 7492–7496.
- 43R. Rajan, K. Matsumura, Sci. Rep. 2017, 7, 45777.
- 44S. Bocian, M. Skoczylas, B. Buszewski, J. Sep. Sci. 2016, 39, 83–92.
- 45L.-J. Xu, C. Zong, X.-S. Zheng, P. Hu, J.-M. Feng, B. Ren, Anal. Chem. 2014, 86, 2238–2245.
- 46E. Podstawka, Y. Ozaki, L. M. Proniewicz, Appl. Spectrosc. 2004, 58, 1147–1156.
- 47Y. Zheng, X. Wang, Y. Ji, Talanta 2012, 91, 7–17.