Phosphine-Catalyzed Difunctionalization of β-Fluoroalkyl α,β-Enones: A Direct Approach to β-Amino α-Diazo Carbonyl Compounds
Huamin Wang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 P. R. China
Search for more papers by this authorLi Zhang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 P. R. China
Search for more papers by this authorYoushao Tu
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 P. R. China
Search for more papers by this authorRuiqi Xiang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Yin-Long Guo
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Junliang Zhang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 P. R. China
Department of Chemistry, Fudan University, 2005 Songhu Road, Shanghai, 200438 P. R. China
Search for more papers by this authorHuamin Wang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 P. R. China
Search for more papers by this authorLi Zhang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 P. R. China
Search for more papers by this authorYoushao Tu
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 P. R. China
Search for more papers by this authorRuiqi Xiang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Yin-Long Guo
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Junliang Zhang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 P. R. China
Department of Chemistry, Fudan University, 2005 Songhu Road, Shanghai, 200438 P. R. China
Search for more papers by this authorDedicated to Professor Xiyan Lu on the occasion of his 90th birthday
Graphical Abstract
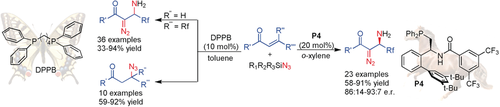
An efficient and practical phosphine-catalyzed vicinal difunctionalization of β-fluoroalkyl α,β-enones with TMSN3 has been developed. Meanwhile, the asymmetry variant induced by the nucleophilic bifunctional phosphine P4 led to various chiral fluoroalkylated β-amino α-diazocarbonyl compounds in high yields and enantioselectivity.
Abstract
An efficient and practical phosphine-catalyzed vicinal difunctionalization of β-fluoroalkyl α,β-enones with TMSN3 has been developed. Using dppb as the catalyst, the reaction worked efficiently to yield various β-amino α-diazocarbonyl compounds in high yields (up to 94 %). This work marks the first efficient construction of α-diazocarbonyl compounds by phosphine catalysis. Meanwhile, the asymmetric variant induced by the nucleophilic bifunctional phosphine P4 led to various chiral fluoroalkylated β-amino α-diazocarbonyl compounds in high yields and enantioselectivity. NMR and ESI-MS studies support the existence of the key reaction intermediates. In contrast, β-azide carbonyl compounds would be furnished in good yields from β-fluoroalkylated β,β-disubstituted enones.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201810253-sup-0001-misc_information.pdf5.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aE. F. V. Scriven, K. Turnbull, Chem. Rev. 1988, 88, 297;
- 1bS. Bräse, C. Gil, K. Knepper, K. Zimmermann, Angew. Chem. Int. Ed. 2005, 44, 5188; Angew. Chem. 2005, 117, 5320;
- 1cA. I. O. Suarez, V. Lyaskovskyy, J. N. H. Reek, J. I. van der Vlugt, B. de Bruin, Angew. Chem. Int. Ed. 2013, 52, 12510; Angew. Chem. 2013, 125, 12740;
- 1dK. Shin, H. Kim, S. Chang, Acc. Chem. Res. 2015, 48, 1040;
- 1eY. Park, Y. Kim, S. Chang, Chem. Rev. 2017, 117, 9247.
- 2
- 2aH. Staudinger, J. Meyer, Helv. Chim. Acta 1919, 2, 635;
- 2bY. G. Gololobov, L. F. Kasukhin, Tetrahedron 1992, 48, 1353;
- 2cJ. E. Leffler, R. D. Temple, J. Am. Chem. Soc. 1967, 89, 5235;
- 2dW. Q. Tian, Y. A. Wang, J. Chem. Theory Comput. 2005, 1, 353;
- 2eL. Horner, A. Gross, Justus Liebigs Ann. Chem. 1955, 591, 117;
- 2fB. L. Nilsson, L. L. Kiessling, R. T. Raines, Org. Lett. 2000, 2, 1939.
- 3
- 3aH. A. van Kalkeren, J. J. Bruins, F. P. J. T. Rutjes, F. L. van Delft, Adv. Synth. Catal. 2012, 354, 1417;
- 3bA. D. Kosal, E. E. Wilson, B. L. Ashfeld, Angew. Chem. Int. Ed. 2012, 51, 12036; Angew. Chem. 2012, 124, 12202;
- 3cK. G. Andrews, R. M. Denton, Chem. Commun. 2017, 53, 7982.
- 4H. H. Chou, R. T. Raines, J. Am. Chem. Soc. 2013, 135, 14936.
- 5E. L. Myers, R. T. Raines, Angew. Chem. Int. Ed. 2009, 48, 2359; Angew. Chem. 2009, 121, 2395.
- 6For reviews, see:
- 6aX. Lu, C. Zhang, Z. Xu, Acc. Chem. Res. 2001, 34, 535;
- 6bJ. L. Methot, W. R. Roush, Adv. Synth. Catal. 2004, 346, 1035;
- 6cL.-W. Ye, J. Zhou, Y. Tang, Chem. Soc. Rev. 2008, 37, 1140;
- 6dB. J. Cowen, S. J. Miller, Chem. Soc. Rev. 2009, 38, 3102;
- 6eA. Marinetti, A. Voituriez, Synlett 2010, 174;
- 6fY. Wei, M. Shi, Acc. Chem. Res. 2010, 43, 1005;
- 6gF. López, J. Mascareñas, Chem. Eur. J. 2011, 17, 418;
- 6hQ.-Y. Zhao, Z. Lian, Y. Wei, M. Shi, Chem. Commun. 2012, 48, 1724;
- 6iY. C. Fan, O. Kwon, Chem. Commun. 2013, 49, 11588;
- 6jZ. Wang, X. Xu, O. Kwon, Chem. Soc. Rev. 2014, 43, 2927;
- 6kP. Xie, Y. Huang, Org. Biomol. Chem. 2015, 13, 8578;
- 6lW. Li, J. Zhang, Chem. Soc. Rev. 2016, 45, 1657;
- 6mT. Wang, X. Han, F. Zhong, W. Yao, Y. Lu, Acc. Chem. Res. 2016, 49, 1369;
- 6nY. Wei, M. Shi, Org. Chem. Front. 2017, 4, 1876.
- 7For some selected examples on phosphine-catalyzed reactions since 2015, see:
- 7aY. Gu, P. Hu, C. Ni, X. Tong, J. Am. Chem. Soc. 2015, 137, 6400;
- 7bL. Zhang, H. Liu, G. Qiao, Z. H. Liu, Y. Xiao, H. Guo, J. Am. Chem. Soc. 2015, 137, 4316;
- 7cS. Lee, Y. Fujiwara, A. Nishi-guchi, M. Kalek, G. Fu, J. Am. Chem. Soc. 2015, 137, 4587;
- 7dE. Li, H. Jin, P. Jia, X. Dong, Y. Huang, Angew. Chem. Int. Ed. 2016, 55, 11591; Angew. Chem. 2016, 128, 11763;
- 7eM. Sankar, M. Castro, C. Golz, C. Strohmann, K. Kumar, Angew. Chem. Int. Ed. 2016, 55, 9709; Angew. Chem. 2016, 128, 9861;
- 7fX. Han, W.-L. Chan, W. Yao, Y. Wang, Y. Lu, Angew. Chem. Int. Ed. 2016, 55, 6492; Angew. Chem. 2016, 128, 6602;
- 7gL. Cai, K. Zhang, O. Kwon, J. Am. Chem. Soc. 2016, 138, 3298;
- 7hB. Satpathi, S. S. V. Ramasastry, Angew. Chem. Int. Ed. 2016, 55, 1777; Angew. Chem. 2016, 128, 1809;
- 7iH.-Y. Wang, C.-W. Zheng, Z. Chai, J.-X. Zhang, G. Zhao, Nat. Commun. 2016, 7, 12720;
- 7jC. Wang, Z. Gao, L. Zhou, C. Yuan, Z. Sun, Y. Xiao, H. Guo, Org. Lett. 2016, 18, 3418;
- 7kH. Ni, X. Tang, W. Zheng, W. Yao, N. Ullah, Y. Lu, Angew. Chem. Int. Ed. 2017, 56, 14222; Angew. Chem. 2017, 129, 14410;
- 7lB. Mao, W. Shi, J. Liao, H. Liu, C. Zhang, H. Guo, Org. Lett. 2017, 19, 6340;
- 7mJ. Chen, Y. Huang, Org. Lett. 2017, 19, 5609.
- 8
- 8aX. Su, W. Zhou, Y. Li, J. Zhang, Angew. Chem. Int. Ed. 2015, 54, 6874; Angew. Chem. 2015, 127, 6978;
- 8bW. Zhou, X. Su, M. Tao, C. Zhu, Q. Zhao, J. Zhang, Angew. Chem. Int. Ed. 2015, 54, 14853; Angew. Chem. 2015, 127, 15066;
- 8cW. Zhou, P. Chen, M. Tao, X. Su, Q. Zhao, J. Zhang, Chem. Commun. 2016, 52, 7612;
- 8dW. Zhou, L, Gao, M. Tao, X. Su, Q. Zhao, J. Zhang, Acta Chim. Sinica 2016, 74, 800;
- 8eP. Chen, Z. Yue, X. Lv, L. Wang, J. Zhang, Angew. Chem. Int. Ed. 2016, 55, 13316; Angew. Chem. 2016, 128, 13510;
- 8fH. Wang, W. Zhou, M. Tao, A. Hu, J. Zhang, Org. Lett. 2017, 19, 1710;
- 8gW. Zhou, H. Wang, M. Tao, C. Zhu, T. Lin, J. Zhang, Chem. Sci. 2017, 8, 4660;
- 8hH. Wang, W. Lu, J. Zhang, Chem. Eur. J. 2017, 23, 13587.
- 9The X-ray crystal structure information is available at the Cambridge Crystallographic Data Centre (CCDC) under deposition number CCDC 1819849 (2 e), 1866246 ((−)-2 e) and 1819850 (3 d).
- 10B. L. Feringa, J. F. G. A. Jansen, Synthesis 1988, 1988, 184.
10.1055/s-1988-27507 Google Scholar
- 11
- 11aM. Saïdouali, M. V. R. Carrié, Tetrahedron 1980, 36, 1821;
- 11bL. Benati, P. C. Montevecchi, J. Chem. Soc. Perkin Trans. 1 1989, 2235.
- 12
- 12aV. B. Di Marco, G. G. Bombi, Mass Spectrom. Rev. 2006, 25, 347;
- 12bK. L. Vikse, Z. Ahmadi, J. S. McIndoe, Coord. Chem. Rev. 2014, 279, 96.