Homo- and Heteroligand Poly-NHC Metal Assemblies: Synthesis by Narcissistic and Social Self-Sorting
Yi-Shou Wang
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry, College of Chemistry and Materials Science, Northwest University, Xi'an, 710127 P. R. China
Search for more papers by this authorTing Feng
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry, College of Chemistry and Materials Science, Northwest University, Xi'an, 710127 P. R. China
Search for more papers by this authorProf. Dr. Yao-Yu Wang
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry, College of Chemistry and Materials Science, Northwest University, Xi'an, 710127 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. F. Ekkehardt Hahn
Institut für Anorganische und Analytische Chemie, Westfälische Wilhelms-Universität Münster, Corrensstrasse 30, 48149 Münster, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Ying-Feng Han
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry, College of Chemistry and Materials Science, Northwest University, Xi'an, 710127 P. R. China
Search for more papers by this authorYi-Shou Wang
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry, College of Chemistry and Materials Science, Northwest University, Xi'an, 710127 P. R. China
Search for more papers by this authorTing Feng
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry, College of Chemistry and Materials Science, Northwest University, Xi'an, 710127 P. R. China
Search for more papers by this authorProf. Dr. Yao-Yu Wang
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry, College of Chemistry and Materials Science, Northwest University, Xi'an, 710127 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. F. Ekkehardt Hahn
Institut für Anorganische und Analytische Chemie, Westfälische Wilhelms-Universität Münster, Corrensstrasse 30, 48149 Münster, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Ying-Feng Han
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry, College of Chemistry and Materials Science, Northwest University, Xi'an, 710127 P. R. China
Search for more papers by this authorGraphical Abstract
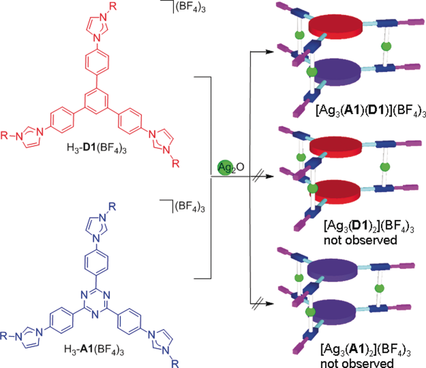
Unprecedented social self-sorting occurs during the reaction of Ag2O with two trisimidazolium salts featuring either an electron-poor H3-A1(BF4)3 or an electron-rich H3-D1(BF4)3 backbone to give the heteroligand assembly [Ag3(A1)(D1)](BF4)3 most likely driven by π⋅⋅⋅π stacking interactions between the electron-rich and electron-poor ligand backbone groups.
Abstract
Homoleptic and heteroleptic cylinder-shaped poly-NHC metallosupramolecular assemblies [Ag3(L)2](BF4)3 have been prepared by control of the shape, size, and electronic properties of disk-shaped trisimidazolium salts of type H3-L(BF4)3. Both imidazolium salts with an electron-deficient triazine backbone H3-A(BF4)3 or an electron-rich benzene backbone H3-D(BF4)3 have been employed. Reaction of H3-A(BF4)3 or H3-D(BF4)3 with Ag2O yield trinuclear homoligand complexes [Ag3(L)2](BF4)3 (L=A, D). However, equimolar mixtures of H3-A(BF4)3 and H3-D(BF4)3 react with Ag2O under social self-sorting to give the heteroligand assembly [Ag3(A)(D)](BF4)3. The same heteroligand assembly was obtained by transmetallation from mixtures of complexes [Ag3(A)2](BF4)3 and [Ag3(D)2](BF4)3. The transmetallation from [Ag3(A)(D)](BF4)3 to [Au3(A)(D)](BF4)3 is also demonstrated. The study expands to concepts of narcissistic and social self-sorting from classical Werner-type ligands to organometallic NHC chemistry thereby opening new routes for the construction of poly-NHC metal assemblies with increasing complexity.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201810010-sup-0001-misc_information.pdf5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. A. Mata, M. Poyatos, E. Peris, Coord. Chem. Rev. 2007, 251, 841–859;
- 1bF. E. Hahn, M. C. Jahnke, Angew. Chem. Int. Ed. 2008, 47, 3122–3172; Angew. Chem. 2008, 120, 3166–3216;
- 1cM. Poyatos, J. A. Mata, E. Peris, Chem. Rev. 2009, 109, 3677–3707;
- 1dL. Mercs, M. Albrecht, Chem. Soc. Rev. 2010, 39, 1903–1912;
- 1eM. Melaimi, M. Soleilhavoup, G. Bertrand, Angew. Chem. Int. Ed. 2010, 49, 8810–8849; Angew. Chem. 2010, 122, 8992–9032;
- 1fB. M. Neilson, A. G. Tennyson, C. W. Bielawski, J. Phys. Org. Chem. 2012, 25, 531–543;
- 1gM. N. Hopkinson, C. Richter, M. Schedler, F. Glorius, Nature 2014, 510, 485–496;
- 1hN. Sinha, F. E. Hahn, Acc. Chem. Res. 2017, 50, 2167–2184;
- 1iM.-M. Gan, J.-Q. Liu, L. Zhang, Y.-Y. Wang, F. E. Hahn, Y.-F. Han, Chem. Rev. 2018, 118, 9587–9641.
- 2
- 2aF. E. Hahn, C. Radloff, T. Pape, A. Hepp, Organometallics 2008, 27, 6408–6410;
- 2bA. Rit, T. Pape, F. E. Hahn, Organometallics 2011, 30, 6393–6401;
- 2cY.-F. Han, G.-X. Jin, F. E. Hahn, J. Am. Chem. Soc. 2013, 135, 9263–9266;
- 2dY.-F. Han, G.-X. Jin, C. G. Daniliuc, F. E. Hahn, Angew. Chem. Int. Ed. 2015, 54, 4958–4962; Angew. Chem. 2015, 127, 5042–5046;
- 2eT. Yan, L.-Y. Sun, Y.-X. Deng, Y.-F. Han, G.-X. Jin, Chem. Eur. J. 2015, 21, 17610–17613.
- 3
- 3aF. M. Conrady, R. Fröhlich, C. Schulte to Brinke, T. Pape, F. E. Hahn, J. Am. Chem. Soc. 2011, 133, 11496–11499;
- 3bM. Schmidtendorf, T. Pape, F. E. Hahn, Angew. Chem. Int. Ed. 2012, 51, 2195–2198; Angew. Chem. 2012, 124, 2238–2241;
- 3cM. Schmidtendorf, T. Pape, F. E. Hahn, Dalton Trans. 2013, 42, 16128–16141;
- 3dN. Sinha, F. Roelfes, A. Hepp, F. E. Hahn, Chem. Eur. J. 2017, 23, 5939–5942.
- 4
- 4aA. Rit, T. Pape, F. E. Hahn, J. Am. Chem. Soc. 2010, 132, 4572–4573;
- 4bD. Wang, B. Zhang, C. He, P. Wu, C. Duan, Chem. Commun. 2010, 46, 4728–4730;
- 4cC. Segarra, G. Guisado-Barrios, F. E. Hahn, E. Peris, Organometallics 2014, 33, 5077–5080;
- 4dN. Sinha, F. Roelfes, A. Hepp, C. Mejuto, E. Peris, F. E. Hahn, Organometallics 2014, 33, 6898–6904;
- 4eP. J. Altmann, A. Pöthig, J. Am. Chem. Soc. 2016, 138, 13171–13174;
- 4fP. J. Altmann, A. Pöthig, Angew. Chem. Int. Ed. 2017, 56, 15733–15736; Angew. Chem. 2017, 129, 15939–15942;
- 4gL.-Y. Sun, N. Sinha, T. Yan, Y.-S. Wang, T. T. Y. Tan, L. Yu, Y.-F. Hahn, F. E. Hahn, Angew. Chem. Int. Ed. 2018, 57, 5161–5165; Angew. Chem. 2018, 130, 5256–5261.
- 5J. C. Y. Lin, R. T. W. Huang, C. S. Lee, A. Bhattacharyya, W. S. Wang, I. J. B. Lin, Chem. Rev. 2009, 109, 3561–3598.
- 6
- 6aN. C. Seeman, Nature 2003, 421, 427–431;
- 6bJ. S. Nowick, D. M. Chung, Angew. Chem. Int. Ed. 2003, 42, 1765–1768; Angew. Chem. 2003, 115, 1807–1810.
- 7
- 7aS. De, K. Mahata, M. Schmittel, Chem. Soc. Rev. 2010, 39, 1555–1575;
- 7bM. L. Saha, S. De, S. Pramanik, M. Schmittel, Chem. Soc. Rev. 2013, 42, 6860–6909;
- 7cM. L. Saha, X. Yan, P. J. Stang, Acc. Chem. Res. 2016, 49, 2527–2539;
- 7dM. M. J. Smulders, I. A. Riddell, C. Browne, J. R. Nitschke, Chem. Soc. Rev. 2013, 42, 1728–1754;
- 7eL.-J. Chen, H.-B. Yang, M. Shionoya, Chem. Soc. Rev. 2017, 46, 2555–2576;
- 7fH. Li, Z.-J. Yao, D. Liu, G.-X. Jin, Coord. Chem. Rev. 2015, 293–294, 139–157;
- 7gY.-F. Han, G.-X. Jin, Acc. Chem. Res. 2014, 47, 3571–3579;
- 7hT. R. Cook, P. J. Stang, Chem. Rev. 2015, 115, 7001–7045;
- 7iG. H. Clever, P. Punt, Acc. Chem. Res. 2017, 50, 2233–2243.
- 8
- 8aA.-M. Stadler, C. Burg, J. Ramírez, J.-M. Lehn, Chem. Commun. 2013, 49, 5733–5735;
- 8bR. Chakrabarty, P. J. Stang, J. Am. Chem. Soc. 2012, 134, 14738–14741;
- 8cT. K. Ronson, D. A. Roberts, S. P. Black, J. R. Nitschke, J. Am. Chem. Soc. 2015, 137, 14502–14512;
- 8dH. Sepehrpour, M. L. Saha, P. J. Stang, J. Am. Chem. Soc. 2017, 139, 2553–2556;
- 8eQ.-F. Sun, S. Sato, M. Fujita, Angew. Chem. Int. Ed. 2014, 53, 13510–13513; Angew. Chem. 2014, 126, 13728–13731;
- 8fG. Jayamurugan, D. A. Roberts, T. K. Ronson, J. R. Nitschke, Angew. Chem. Int. Ed. 2015, 54, 7539–7543; Angew. Chem. 2015, 127, 7649–7653.
- 9
- 9aS. Hiraoka, M. Shiro, M. Shionoya, J. Am. Chem. Soc. 2004, 126, 1214–1218;
- 9bW. M. Bloch, Y. Abe, J. J. Holstein, C. M. Wandtke, B. Dittrich, G. H. Clever, J. Am. Chem. Soc. 2016, 138, 13750–13755;
- 9cW. M. Bloch, J. J. Holstein, W. Hiller, G. H. Clever, Angew. Chem. Int. Ed. 2017, 56, 8285–8289; Angew. Chem. 2017, 129, 8399–8404.
- 10
- 10aS.-L. Huang, Y.-J. Lin, T. S. A. Hor, G.-X. Jin, J. Am. Chem. Soc. 2013, 135, 8125–8128;
- 10bW.-Y. Zhang, Y.-J. Lin, Y.-F. Han, G.-X. Jin, J. Am. Chem. Soc. 2016, 138, 10700–10707;
- 10cD. Preston, J. E. Barnsley, K. C. Gordon, J. D. Crowley, J. Am. Chem. Soc. 2016, 138, 10578–10585;
- 10dJ. Jiao, C. Tan, Z. Li, Y. Liu, X. Han, Y. Cui, J. Am. Chem. Soc. 2018, 140, 2251–2259;
- 10eL. Zhang, L. Lin, D. Liu, Y.-J. Lin, Z.-H. Li, G.-X. Jin, J. Am. Chem. Soc. 2017, 139, 1653–1660;
- 10fS.-L. Huang, T. S. A. Hor, G.-X. Jin, Coord. Chem. Rev. 2017, 333, 1–26;
- 10gY. Lu, H.-N. Zhang, G.-X. Jin, Acc. Chem. Res. 2018, 51, 2148–2158.
- 11
- 11aC. M. Crudden, D. P. Allen, Coord. Chem. Rev. 2004, 248, 2247–2273;
- 11bH. Jacobsen, A. Correa, A. Poater, C. Costabile, L. Cavallo, Coord. Chem. Rev. 2009, 253, 687–703.
- 12
- 12aN. Sinha, T. T. Y. Tan, E. Peris, F. E. Hahn, Angew. Chem. Int. Ed. 2017, 56, 7393–7397; Angew. Chem. 2017, 129, 7499–7503.
- 13C. Mejuto, G. Guisado-Barrios, D. Gusev, E. Peris, Chem. Commun. 2015, 56, 7393–7397.
- 14
- 14aS. R. Lokey, B. L. Iverson, Nature 1995, 375, 303–305;
- 14bT. Ono, M. Sugimoto, Y. Hisaeda, J. Am. Chem. Soc. 2015, 137, 9519–9522;
- 14cB. Zhao, N. Li, X. Wang, Z. Chang, X.-H. Bu, ACS Appl. Mater. Interfaces 2017, 9, 2662–2668.