Valve-Enabled Sample Preparation and RNA Amplification in a Coffee Mug for Zika Virus Detection
Dr. Xiao Jiang
J. Crayton Pruitt Family Department of Biomedical Engineering, University of Florida, Gainesville, FL, 32611 USA
Search for more papers by this authorJulia C. Loeb
Department of Environmental and Global Health, and Emerging Pathogens Institute, University of Florida, Gainesville, FL, 32611 USA
Search for more papers by this authorCarlos Manzanas
Department of Mechanical and Aerospace Engineering, University of Florida, P.O. Box 116250, Gainesville, FL, 32611 USA
Search for more papers by this authorProf. John A. Lednicky
Department of Environmental and Global Health, and Emerging Pathogens Institute, University of Florida, Gainesville, FL, 32611 USA
Search for more papers by this authorCorresponding Author
Prof. Z. Hugh Fan
J. Crayton Pruitt Family Department of Biomedical Engineering, University of Florida, Gainesville, FL, 32611 USA
Department of Mechanical and Aerospace Engineering, University of Florida, P.O. Box 116250, Gainesville, FL, 32611 USA
Search for more papers by this authorDr. Xiao Jiang
J. Crayton Pruitt Family Department of Biomedical Engineering, University of Florida, Gainesville, FL, 32611 USA
Search for more papers by this authorJulia C. Loeb
Department of Environmental and Global Health, and Emerging Pathogens Institute, University of Florida, Gainesville, FL, 32611 USA
Search for more papers by this authorCarlos Manzanas
Department of Mechanical and Aerospace Engineering, University of Florida, P.O. Box 116250, Gainesville, FL, 32611 USA
Search for more papers by this authorProf. John A. Lednicky
Department of Environmental and Global Health, and Emerging Pathogens Institute, University of Florida, Gainesville, FL, 32611 USA
Search for more papers by this authorCorresponding Author
Prof. Z. Hugh Fan
J. Crayton Pruitt Family Department of Biomedical Engineering, University of Florida, Gainesville, FL, 32611 USA
Department of Mechanical and Aerospace Engineering, University of Florida, P.O. Box 116250, Gainesville, FL, 32611 USA
Search for more papers by this authorGraphical Abstract
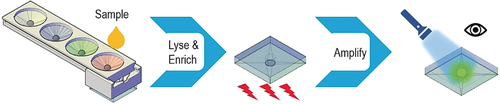
POC Zika detection: A valve-enabled lysis, paper-based RNA enrichment, and RNA amplification device (VLEAD) is designed for sample-to-answer Zika virus detection. RNA is enriched with VLEAD in 25 min. A colorimetric signal is generated with SYBR Green dye and a blue LED flashlight after 25 min of reverse transcription loop-mediated amplification (RT-LAMP).
Abstract
The recent outbreaks of Zika virus (ZIKV) infection represent a public health challenge. Rapid, cost-effective, and reliable diagnostic tools for ZIKV detection at the point of care (POC) are highly desirable, especially for resource-limited nations. To address the need, we have developed an integrated device to achieve sample-to-answer ZIKV detection. The device features innovative ball-based valves enabling the storage and sequential delivery of reagents for virus lysis and a paper-based unit for RNA enrichment and purification. The paper unit is placed in a commercially available coffee mug that provides a constant temperature for reverse transcription loop-mediated isothermal amplification (RT-LAMP), followed by colorimetric detection by naked eye or a cellphone camera. Using the device, we demonstrated the reproducible detection of ZIKV in human urine and saliva samples.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201809993-sup-0001-misc_information.pdf1.3 MB | Supplementary |
| anie201809993-sup-0001-VideoS1.mp41.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Á. Fajardo, J. Cristina, P. Moreno, Front. Microbiol. 2016, 7, 1667.
- 2CDC, “Zika Cases in the United States” can be found under https://www.cdc.gov/zika/reporting/case-counts.html.
- 3D. Musso, D. J. Gubler, Clin. Microbiol. Rev. 2016, 29, 487.
- 4CDC, “Testing Guidance” can be found under https://www.cdc.gov/zika/hc-providers/testing-guidance.html.
- 5Z. K. Njiru, D. J. Gubler, PLoS Neglected Trop. Dis. 2012, 6, e 1572.
- 6M. Safavieh, M. K. Kanakasabapathy, F. Tarlan, M. U. Ahmed, M. Zourob, W. Asghar, H. Shafiee, ACS Biomater. Sci. Eng. 2016, 2, 278.
- 7A. Priye, S. W. Bird, Y. K. Light, C. S. Ball, O. A. Negrete, R. J. Meagher, Sci. Rep. 2017, 7, 44778.
- 8N. Chotiwan, C. D. Brewster, T. Magalhaes, J. Weger-Lucarelli, N. K. Duggal, C. Rückert, C. Nguyen, S. M. G. Luna, J. R. Fauver, B. Adre, M. Gray, Sci. Transl. Med. 2017, 9, eaag 0538.
- 9A. Ganguli, A. Ornob, H. Yu, G. L. Damhorst, W. Chen, F. Sun, A. Bhuiya, B. T. Cunningham, R. Bashir, Biomed. Microdevices 2017, 19, 73.
- 10X. Wang, F. Yin, Y. Bi, G. Cheng, J. Li, L. Hou, Y. Li, B. Yang, W. Liu, L. Yang, J. Virol. Methods 2016, 238, 86.
- 11A. E. Calvert, B. J. Biggerstaff, N. A. Tanner, M. Lauterbach, R. S. Lanciotti, PloS One 2017, 12, e 0185340.
- 12Y. Kurosaki, D. B. G. Martins, M. Kimura, A. D. S. Catena, M. A. C. S. M. Borba, S. D. S. Mattos, H. Abe, R. Yoshikawa, J. L. de Lima Filho, Sci. Rep. 2017, 7, 13503.
- 13M. Sabalza, R. Yasmin, C. A. Barber, T. Castro, D. Malamud, B. J. Kim, H. Zhu, R. A. Montagna, W. R. Abrams, PloS One 2018, 13, e 0192398.
- 14I. Bosch, H. de Puig, M. Hiley, M. Carré-Camps, F. Perdomo-Celis, C. F. Narváez, D. M. Salgado, D. Senthoor, M. O'Grady, E. Phillips, A. Durbin, Sci. Transl. Med. 2017, 9, eaan 1589.
- 15Y. Lustig, D. Sofer, M. Hindiyeh, E. Mendelson, Ann. Transl. Med. 2018, 6, 53.
- 16C. Liu. M. G. Mauk, R. Hart, M. Bonizzoni, G. Yan, H. H. Bau, PloS One 2012, 7, e 42222.
- 17A. V. Govindarajan, S. Ramachandran, G. D. Vigil, P. Yager, K. F. Böhringer, Lab Chip 2012, 12, 174.
- 18L. Zhang, Y. Zhang, C. Wang, Q. Feng, F. Fan, G. Zhang, X. Kang, X. Qin, J. Sun, Y. Li, X. Jiang, Anal. Chem. 2014, 86, 10461.
- 19R. Wimbles, L. M. Melling, K. J. Shaw, Micromachines 2016, 7, 119.
- 20C. Liu, E. Geva, M. Mauk, X. Qiu, W. R. Abrams, D. Malamud, K. Curtis, S. M. Owen, H. H. Bau, Analyst 2011, 136, 2069.
- 21J. R. Choi, K. W. Yong, R. Tang, Y. Gong, T. Wen, H. Yang, A. Li, Y. C. Chia, B. Pingguan-Murphy, F. Xu, Adv. Healthcare Mater. 2017, 6, 1600920.
- 22J. T. Connelly, J. P. Rolland, G. M. Whitesides, Anal. Chem. 2015, 87, 7595.
- 23B. Alberts, G. Herrick, Methods Enzymol. 1971, 21, 198.
- 24S. A. Byrnes, J. D. Bishop, L. Lafleur, J. R. Buser, B. Lutz, P. Yager, Lab Chip 2015, 15, 2647.
- 25X. Ye, J. Xu, L. Lu, X. Li, X. Fang, J. Kong, Anal. Chim. Acta 2018, 1018, 78.
- 26J. Song, M. G. Mauk, B. A. Hackett, S. Cherry, H. H. Bau, C. Liu, Anal. Chem. 2016, 88, 7289.
- 27G. Xu, D. Nolder, J. Reboud, M. C. Oguike, D. A. van Schalkwyk, C. J. Sutherland, J. M. Cooper, Angew. Chem. Int. Ed. 2016, 55, 15250; Angew. Chem. 2016, 128, 15476.
- 28R. Tang, H. Yang, Y. Gong, M. You, Z. Liu, J. R. Choi, T. Wen, Z. Qu, Q. Mei, F. Xu, Lab Chip 2017, 17, 1270.
- 29N. M. Rodriguez, J. C. Linnes, A. Fan, C. K. Ellenson, N. R. Pollock, C. M. Klapperich, Anal. Chem. 2015, 87, 7872.
- 30N. M. Rodriguez, W. S. Wong, L. Liu, R. Dewar, C. M. Klapperich, Lab Chip 2016, 16, 753.
- 31A. L. Horst, J. M. Rosenbohm, N. Kolluri, J. Hardick, C. A. Gaydos, M. Cabodi, C. M. Klapperich, J. C. Linnes, J. Biomed. Microdevices 2018, 20, 35.
- 32A. W. Martinez, S. T. Phillips, M. J. Butte, G. M. Whitesides, Angew. Chem. Int. Ed. 2007, 46, 1318; Angew. Chem. 2007, 119, 1340.
- 33W. Liu, C. L. Cassano, X. Xu, Z. H. Fan, Anal. Chem. 2013, 85, 10270.
- 34C. L. Cassano, Z. H. Fan, Microfluid. Nanofluid. 2013, 15, 173.
- 35N. Kaur, B. J. Toley, Analyst 2018, 143, 2213.
- 36P. Sairkar, S. Chouhan, N. Batav, R. J. Sharma, Genet. Eng. Biotechnol. 2013, 11, 17.
10.1016/j.jgeb.2013.02.001 Google Scholar
- 37X. Jiang, J. Loeb, M. Pan, T. B. Tilly, J. A. Lednicky, C. Y. Wu, Z. H. Fan, unpublished work.
- 38T. Iwamoto, T. Sonobe, K. Hayashi, J. Clin. Microbiol. 2003, 41, 2616.
- 39M. T. Koesdjojo, S. Pengpumkiat, Y. Wu, A. Boonloed, D. Huynh, T. P. Remcho, V. T. Remcho, J. Chem. Educ. 2015, 92, 737.
- 40A. M. Bingham, “Comparison of Test Results for ZIKV RNA in Urine, Serum, and Saliva Specimens from Persons with Travel-Associated ZIKV Disease—Florida, 2016.” can be found under https://www.cdc.gov/mmwr/volumes/65/wr/mm6518e2.htm.
- 41G. Paz-Bailey, E. S. Rosenberg, K. Doyle, J. Munoz-Jordan, G. A. Santiago, L. Klein, J. Perez-Padilla, F. A. Medina, S. H. Waterman, C. G. Gubern, L. I. Alvarado, N. Engl. J. Med. 2017, https://doi.org/10.1056/NEJMoa1613108.
- 42A. Fontaine, F. de Laval, D. Belleoud, S. Briolant, S. Matheus, Clin. Infect. Dis. 2018, https://doi.org/10.1093/cid/ciy261.
- 43J. Cohen, Science 2017, 357, 631.