Segmental 13C-Labeling and Raman Microspectroscopy of α-Synuclein Amyloid Formation
Graphical Abstract
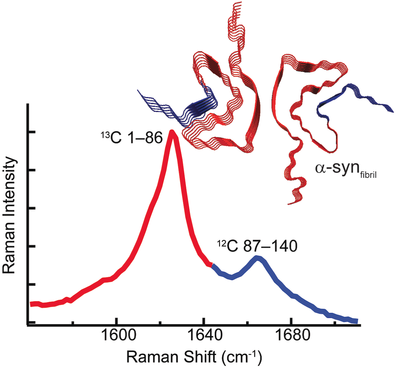
Two peaks are better than one: Native chemical ligation was used to segmentally label α-synuclein with 13C. This labeling results in two spectroscopically distinct amide-I bands that independently report on region-specific conformational changes of the protein backbone. The N-terminal region was seen to adopt a β-sheet structure first in the formation of amyloid.
Abstract
Mapping conformational changes of α-synuclein (α-syn) from soluble, unstructured monomers to β-sheet- rich aggregates is crucial towards understanding amyloid formation. Raman microspectroscopy is now used to spatially resolve conformational heterogeneity of amyloid aggregates and monitor amyloid formation of segmentally 13C-labeled α-syn in real-time. As the 13C-isotope shifts the amide-I stretching frequency to lower energy, the ligated construct, 13C1–8612CS87C–140-α-syn, exhibits two distinct bands allowing for simultaneous detection of secondary structural changes in N-terminal 1–86 and C-terminal 87–140 residues. The disordered-to-β-sheet conformational change is first observed for the N-terminal followed by the C-terminal region. Finally, Raman spectroscopic changes occurred prior to Thioflavin T fluorescence enhancement, indicating that the amide-I band is a superior probe of amyloid formation.