A General Amino Acid Synthesis Enabled by Innate Radical Cross-Coupling
Shengyang Ni
Scripps Research, North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorAlberto F. Garrido-Castro
Scripps Research, North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorRohan R. Merchant
Scripps Research, North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorJustine N. de Gruyter
Scripps Research, North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorDr. Daniel C. Schmitt
Pfizer Medicinal Sciences, Eastern Point Road, Groton, CT, 06340 USA
Search for more papers by this authorDr. James J. Mousseau
Pfizer Medicinal Sciences, Eastern Point Road, Groton, CT, 06340 USA
Search for more papers by this authorDr. Gary M. Gallego
Department of Chemistry, La Jolla Laboratories, Pfizer, 10770 Science Center Drive, San Diego, CA, 92121 USA
Search for more papers by this authorDr. Shouliang Yang
Department of Chemistry, La Jolla Laboratories, Pfizer, 10770 Science Center Drive, San Diego, CA, 92121 USA
Search for more papers by this authorMichael R. Collins
Department of Chemistry, La Jolla Laboratories, Pfizer, 10770 Science Center Drive, San Diego, CA, 92121 USA
Search for more papers by this authorDr. Jennifer X. Qiao
Department of Discovery Chemistry, Bristol-Myers Squibb Company, Research and Development, P.O. Box 4000, Princeton, NJ, 08543 USA
Search for more papers by this authorDr. Kap-Sun Yeung
Department of Discovery Chemistry, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT, 06492 USA
Search for more papers by this authorDr. David R. Langley
Department of Discovery Chemistry, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT, 06492 USA
Search for more papers by this authorDr. Michael A. Poss
Department of Discovery Chemistry, Bristol-Myers Squibb Company, Research and Development, P.O. Box 4000, Princeton, NJ, 08543 USA
Search for more papers by this authorDr. Paul M. Scola
Department of Discovery Chemistry, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT, 06492 USA
Search for more papers by this authorDr. Tian Qin
Scripps Research, North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorCorresponding Author
Prof. Phil S. Baran
Scripps Research, North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorShengyang Ni
Scripps Research, North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorAlberto F. Garrido-Castro
Scripps Research, North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorRohan R. Merchant
Scripps Research, North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorJustine N. de Gruyter
Scripps Research, North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorDr. Daniel C. Schmitt
Pfizer Medicinal Sciences, Eastern Point Road, Groton, CT, 06340 USA
Search for more papers by this authorDr. James J. Mousseau
Pfizer Medicinal Sciences, Eastern Point Road, Groton, CT, 06340 USA
Search for more papers by this authorDr. Gary M. Gallego
Department of Chemistry, La Jolla Laboratories, Pfizer, 10770 Science Center Drive, San Diego, CA, 92121 USA
Search for more papers by this authorDr. Shouliang Yang
Department of Chemistry, La Jolla Laboratories, Pfizer, 10770 Science Center Drive, San Diego, CA, 92121 USA
Search for more papers by this authorMichael R. Collins
Department of Chemistry, La Jolla Laboratories, Pfizer, 10770 Science Center Drive, San Diego, CA, 92121 USA
Search for more papers by this authorDr. Jennifer X. Qiao
Department of Discovery Chemistry, Bristol-Myers Squibb Company, Research and Development, P.O. Box 4000, Princeton, NJ, 08543 USA
Search for more papers by this authorDr. Kap-Sun Yeung
Department of Discovery Chemistry, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT, 06492 USA
Search for more papers by this authorDr. David R. Langley
Department of Discovery Chemistry, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT, 06492 USA
Search for more papers by this authorDr. Michael A. Poss
Department of Discovery Chemistry, Bristol-Myers Squibb Company, Research and Development, P.O. Box 4000, Princeton, NJ, 08543 USA
Search for more papers by this authorDr. Paul M. Scola
Department of Discovery Chemistry, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT, 06492 USA
Search for more papers by this authorDr. Tian Qin
Scripps Research, North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorCorresponding Author
Prof. Phil S. Baran
Scripps Research, North Torrey Pines Road, La Jolla, CA, 92037 USA
Search for more papers by this authorGraphical Abstract
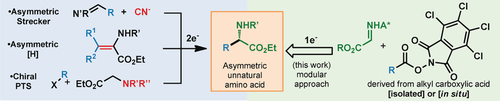
Acids to acids: A practical method for the preparation of highly valuable enantiomerically pure α-amino acids has been developed. The described transformation rapidly converts feedstock alkyl carboxylic acids to α-amino acids, and is enabled by innate radical cross-coupling with a chiral imine radical acceptor. The scope of this transformation is broad and has been field-tested in three different industrial medicinal chemistry laboratories.
Abstract
The direct union of primary, secondary, and tertiary carboxylic acids with a chiral glyoxylate-derived sulfinimine provides rapid access into a variety of enantiomerically pure α-amino acids (>85 examples). Characterized by operational simplicity, this radical-based reaction enables the modular assembly of exotic α-amino acids, including both unprecedented structures and those of established industrial value. The described method performs well in high-throughput library synthesis, and has already been implemented in three distinct medicinal chemistry campaigns.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201809310-sup-0001-misc_information.pdf7.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. Strecker, Ann. Chem. Pharm. 1850, 75, 27.
10.1002/jlac.18500750103 Google Scholar
- 2For a recent review on asymmetric Strecker reactions, see: J. Wang, X. Liu, X. Feng, Chem. Rev. 2011, 111, 6947.
- 3W. S. Knowles, M. J. Sabacky, Chem. Commun. 1968, 1445.
- 4
- 4aW. S. Knowles—Nobel Lecture: Asymmetric Hydrogenations, Nobelprize.org, Nobel Media AB 2014;
- 4bW. S. Knowles, Angew. Chem. Int. Ed. 2002, 41, 1998; Angew. Chem. 2002, 114, 2096.
- 5For additional asymmetric hydrogenations leading to enantiopure AAs, see:
- 5aA. Miyashita, A. Yasuda, H. Takaya, K. Toriumi, T. Ito, T. Souchi, R. Noyori, J. Am. Chem. Soc. 1980, 102, 7932;
- 5bM. J. Burk, J. E. Feaster, W. A. Nugent, R. L. Harlow, J. Am. Chem. Soc. 1993, 115, 10125;
- 5cM. J. Burk, M. F. Gross, J. P. Martinez, J. Am. Chem. Soc. 1995, 117, 9375.
- 6For reviews on phase-transfer catalysis in AA synthesis, see:
- 6aK. Maruoka, T. Ooi, Chem. Rev. 2003, 103, 3013;
- 6bM. J. O'Donnell, Acc. Chem. Res. 2004, 37, 506.
- 7For an elegant example to access α-amino acids through the asymmetric Strecker reaction, see: S. J. Zuend, M. P. Coughlin, M. P. Ladonde, E. N. Jacobsen, Nature 2009, 461, 968.
- 8
- 8aFor review on the role of Ellman's sulfinamide in AA synthesis, see section 9 in: M. T. Robak, M. A. Herbage, J. A. Ellman, Chem. Rev. 2010, 110, 3600;
- 8bF. A. Davis, P. S. Portonovo, R. E. Reddy, Y. Chiu, J. Org. Chem. 1996, 61, 440;
- 8cF. A. Davis, W. McCoull, J. Org. Chem. 1999, 64, 3396;
- 8dF. A. Davis, S. Lee, H. Zhang, D. L. Fanelli, J. Org. Chem. 2000, 65, 8704;
- 8eS. Mabic, A. A. Cordi, Tetrahedron 2001, 57, 8861;
- 8fG. Borg, M. Chino, J. A. Ellman, Tetrahedron Lett. 2001, 42, 1433;
- 8gM. A. Beenen, D. J. Weix, J. A. Ellman, J. Am. Chem. Soc. 2006, 128, 6304;
- 8hH. Wang, X. Zhao, Y. Li, L. Lu, Org. Lett. 2006, 8, 1379.
- 9For a review on the use of radical additions in chiral amine synthesis, see: G. K. Friestad, Top. Curr. Chem. 2014, 343, 1.
- 10For reviews on the importance of unnatural AAs, see:
- 10aC. T. Walsh, R. V. O'Brien, C. Khosla, Angew. Chem. Int. Ed. 2013, 52, 7098; Angew. Chem. 2013, 125, 7238;
- 10bK. Lang, J. W. Chin, Chem. Rev. 2014, 114, 4764;
- 10cM. A. Blaskovich, J. Med. Chem. 2016, 59, 10807.
- 11E. J. Corey, X. M. Cheng, The Logic of Chemical Synthesis, Wiley, New York, 1989.
- 12For reviews on radical-based strategies in synthesis, see:
- 12aM. Yan, J. C. Lo, J. T. Edwards, P. S. Baran, J. Am. Chem. Soc. 2016, 138, 12692;
- 12bJ. M. Smith, S. J. Harwood, P. S. Baran, Acc. Chem. Res. 2018, https://doi.org/10.1021/acs.accounts.8b00209.
- 13For asymmetric radical additions to imines, see:
- 13aY.-W. Zhong, M.-H. Xu, G.-Q. Lin, Org. Lett. 2004, 6, 3953;
- 13bY.-W. Zhong, K. Izumi, M.-H. Xu, G.-Q. Lin, Org. Lett. 2004, 6, 4747;
- 13cY.-W. Zhong, Y.-Z. Dong, K. Fang, K. Izumi, M.-H. Xu, G.-Q. Lin, J. Am. Chem. Soc. 2005, 127, 11956;
- 13dT. Akindele, Y. Yamamoto, M. Maekawa, H. Umeki, K.-I. Yamada, K. Tomioka, Org. Lett. 2006, 8, 5729;
- 13eJ. A. Fernández-Salas, M. C. Maestro, M. M. Rodríguez-Fernández, J. L. García-Ruano, I. Alonso, Org. Lett. 2013, 15, 1658;
- 13fJ. A. Fernández-Salas, M. M. Rodríguez-Fernández, M. C. Maestro, J. L. García-Ruano, Eur. J. Org. Chem. 2014, 5265;
- 13gD. Uraguchi, N. Kinoshita, T. Kizu, T. Ooi, J. Am. Chem. Soc. 2015, 137, 13768;
- 13hT. Kizu, D. Uraguchi, T. Ooi, J. Org. Chem. 2016, 81, 6953;
- 13iA. F. Garrido-Castro, H. Choubane, M. Daaou, M. C. Maestro, J. Alemán, Chem. Commun. 2017, 53, 7764.
- 14See the Supporting Information for details.
- 15
- 15aS. Abele, D. Seebach, Eur. J. Org. Chem. 2000, 1;
- 15bK. R. Heard, W. Wu, Y. Li, P. Zhao, I. Woznica, J. H. Lai, M. Beinborn, D. G. Sanford, M. T. Dimare, A. K. Chiluwal, D. E. Peters, D. Whicher, J. L. Sudmeier, W. W. Bachovchin, J. Med. Chem. 2013, 56, 8339.
- 16M. R. Myers, W. He, B. Hanney, N. Setzer, M. P. Maguire, A. Zulli, G. Bilder, H. Galzcinski, D. Amin, S. Needle, A. P. Spada, Bioorg. Med. Chem. Lett. 2003, 13, 3091.
- 17D. Bandak, O. Babii, R. Vasiuta, I. V. Komarov, P. K. Mykhailiuk, Org. Lett. 2015, 17, 226.
- 18J. Wlochal, R. D. M. Davies, J. Burton, Synlett 2016, 27, 919.
- 19
- 19aR. Pellicciari, M. Raimondo, M. Marinozzi, B. Natalini, G. Costantino, C. Thomsen, J. Med. Chem. 1996, 39, 2874;
- 19bG. Costantino, K. Maltoni, M. Marinozzi, E. Camaioni, L. Prezeau, J.-P. Pin, R. Pellicciari, Bioorg. Med. Chem. 2001, 9, 221;
- 19cP. K. Mikhailiuk, S. Afonin, A. N. Chernega, E. B. Rusanov, M. O. Platonov, G. G. Dubinina, M. Berditsch, A. S. Ulrich, I. V. Komarov, Angew. Chem. Int. Ed. 2006, 45, 5659; Angew. Chem. 2006, 118, 5787;
- 19dR. Filosa, M. Marinozzi, G. Costantino, M. B. Hermit, C. Thomsen, R. Pellicciari, Bioorg. Med. Chem. 2006, 14, 3811;
- 19eS. Pritz, M. Pätzel, G. Szeimies, M. Dathe, M. Bienert, Org. Biomol. Chem. 2007, 5, 1789;
- 19fR. Filosa, M. C. Fulco, M. Marinozzi, N. Giacchè, A. Macchiarulo, A. Peduto, A. Massa, P. de Caprariis, C. Thomsen, C. T. Christoffersen, R. Pellicciari, Bioorg. Med. Chem. 2009, 17, 242;
- 19gP. K. Mykhailiuk, N. M. Voievoda, S. Afonin, A. S. Ulrich, I. V. Komarov, J. Fluorine Chem. 2010, 131, 217;
- 19hS. O. Kokhan, A. V. Tymtsunik, S. L. Grage, S. Afonin, O. Babii, M. Berditsch, A. V. Strizhak, D. Bandak, M. O. Platonov, I. V. Komarov, A. S. Ulrich, P. K. Mykhailiuk, Angew. Chem. Int. Ed. 2016, 55, 14788; Angew. Chem. 2016, 128, 15008.
- 20
- 20aJ. Cornella, J. T. Edwards, T. Qin, S. Kawamura, J. Wang, C.-M. Pan, R. Gianatassio, M. Schmidt, M. D. Eastgate, P. S. Baran, J. Am. Chem. Soc. 2016, 138, 2174;
- 20bT. Qin, J. Cornella, C. Li, L. R. Malins, J. T. Edwards, S. Kawamura, B. D. Maxwell, M. D. Eastgate, P. S. Baran, Science 2016, 352, 801;
- 20cJ. Wang, T. Qin, T.-G. Chen, L. Wimmer, J. T. Edwards, J. Cornella, B. Vokits, S. A. Shaw, P. S. Baran, Angew. Chem. Int. Ed. 2016, 55, 9676; Angew. Chem. 2016, 128, 9828;
- 20dF. Toriyama, J. Cornella, L. Wimmer, T.-G. Chen, D. D. Dixon, G. Creech, P. S. Baran, J. Am. Chem. Soc. 2016, 138, 11132;
- 20eT. Qin, L. R. Malins, J. T. Edwards, R. R. Merchant, A. J. E. Novak, J. Z. Zhong, R. B. Mills, M. Yan, C. Yuan, M. D. Eastgate, P. S. Baran, Angew. Chem. Int. Ed. 2017, 56, 260; Angew. Chem. 2017, 129, 266;
- 20fF. Sandfort, M. J. O'Neill, J. Cornella, L. Wimmer, P. S. Baran, Angew. Chem. Int. Ed. 2017, 56, 3319; Angew. Chem. 2017, 129, 3367;
- 20gJ. T. Edwards, R. R. Merchant, K. S. McClymont, K. W. Knouse, T. Qin, L. R. Malins, B. Vokits, S. A. Shaw, D.-H. Bao, F.-L. Wei, T. Zhou, M. D. Eastgate, P. S. Baran, Nature 2017, 545, 213;
- 20hJ. Smith, T. Qin, R. R. Merchant, J. T. Edwards, L. R. Malins, Z. Liu, G. Che, Z. Shen, S. A. Shaw, M. D. Eastgate, P. S. Baran, Angew. Chem. Int. Ed. 2017, 56, 11906; Angew. Chem. 2017, 129, 12068. For other example of using RAE cross-coupling, see:
- 20iK. M. M. Huihui, J. A. Caputo, Z. Melchor, A. M. Olivares, A. M. Spiewak, K. A. Johnson, T. A. DiBenedetto, S. Kim, L. K. G. Ackerman, D. J. Weix, J. Am. Chem. Soc. 2016, 138, 5016. For a seminal use of RAEs of the phthalimide-type, see:
- 20jK. Okada, K. Okamoto, M. Oda, J. Am. Chem. Soc. 1988, 110, 8736.
- 21X.-L. Qiu, F.-L. Qing, Eur. J. Org. Chem. 2011, 3261.
- 22
- 22aA. W. Buesking, T. D. Baguley, J. A. Ellman, Org. Lett. 2011, 13, 964;
- 22bSee the Supporting Information for control studies (compound 63 a).
- 23
- 23aB. J. Backes, D. R. Dragoli, J. A. Ellman, J. Org. Chem. 1999, 64, 5472. Enantiopure mesitylsulfinamide synthesis, see:
- 23bT. Ramachandar, Y. Wu, J. Zhang, F. A. Davis, Org. Synth. 2006, 83, 131.
- 24Examples of parallel synthesis of homochiral β-amino acids, see: S. G. Davies, A. W. Mulvaney, A. J. Russell, A. D. Smith, Tetrahedron: Asymmetry 2007, 18, 1554.
- 25 Priviledged scaffolds in medicinal chemistry:design, synthesis, evaluation (Ed.: ), RSC, London, 2015.
- 26C. W. Murray, D. C. Rees, Nat. Chem. 2009, 1, 187.
- 27CCDC 1861500and 1861501 (3 and 50) contains the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre.