Electrophilic Phosphonium Cation-Mediated Phosphane Oxide Reduction Using Oxalyl Chloride and Hydrogen
Arne J. Stepen
Department of Chemistry, University of Paderborn, Warburger Strasse 100, 33098 Paderborn, Germany
Department of Chemistry, University of Toronto, 80 St. George St, Toronto, Ontario, M5S3H6 Canada
Search for more papers by this authorMarkus Bursch
Mulliken Center for Theoretical Chemistry, University of Bonn, Beringstr. 4, 53115 Bonn, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Stefan Grimme
Mulliken Center for Theoretical Chemistry, University of Bonn, Beringstr. 4, 53115 Bonn, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Douglas W. Stephan
Department of Chemistry, University of Toronto, 80 St. George St, Toronto, Ontario, M5S3H6 Canada
Search for more papers by this authorCorresponding Author
Prof. Dr. Jan Paradies
Department of Chemistry, University of Paderborn, Warburger Strasse 100, 33098 Paderborn, Germany
Search for more papers by this authorArne J. Stepen
Department of Chemistry, University of Paderborn, Warburger Strasse 100, 33098 Paderborn, Germany
Department of Chemistry, University of Toronto, 80 St. George St, Toronto, Ontario, M5S3H6 Canada
Search for more papers by this authorMarkus Bursch
Mulliken Center for Theoretical Chemistry, University of Bonn, Beringstr. 4, 53115 Bonn, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Stefan Grimme
Mulliken Center for Theoretical Chemistry, University of Bonn, Beringstr. 4, 53115 Bonn, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Douglas W. Stephan
Department of Chemistry, University of Toronto, 80 St. George St, Toronto, Ontario, M5S3H6 Canada
Search for more papers by this authorCorresponding Author
Prof. Dr. Jan Paradies
Department of Chemistry, University of Paderborn, Warburger Strasse 100, 33098 Paderborn, Germany
Search for more papers by this authorGraphical Abstract
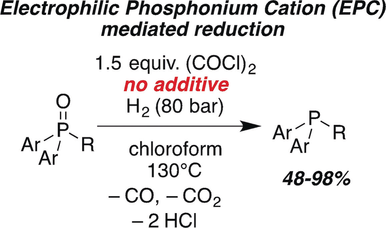
The metal-free reduction of phosphane oxides with H2 using oxalyl chloride as activating agent was achieved. H2 is activated by the in situ formed electrophilic phosphonium cation and phosphane oxide. The reaction is also catalyzed by B(2,6-F2C6H3)3 and 2,6-lutidine or phosphane oxide as Lewis base.
Abstract
The metal-free reduction of phosphane oxides with molecular hydrogen (H2) using oxalyl chloride as activating agent was achieved. Quantum-mechanical investigations support the heterolytic splitting of H2 by the in situ formed electrophilic phosphonium cation (EPC) and phosphane oxide and subsequent barrierless conversion to the phosphane and HCl. The reaction can also be catalyzed by the frustrated Lewis pair (FLP) consisting of B(2,6-F2C6H3)3 and 2,6-lutidine or phosphane oxide as Lewis base. This novel reduction was demonstrated for triaryl and diaryl phosphane oxides providing access to phosphanes in good to excellent yields (51–93 %).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201809275-sup-0001-misc_information.pdf2.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. Eggersdorfer, D. Laudert, U. Letinois, T. McClymont, J. Medlock, T. Netscher, W. Bonrath, Angew. Chem. Int. Ed. 2012, 51, 12960–12990; Angew. Chem. 2012, 124, 13134–13165.
- 2
- 2aL. Horner, W. D. Balzer, Tetrahedron Lett. 1965, 6, 1157–1162;
10.1016/S0040-4039(01)83990-3 Google Scholar
- 2bN. L. Bauld, F. Farr, J. Am. Chem. Soc. 1969, 91, 2788–2788;
- 2cR. Tang, K. Mislow, J. Am. Chem. Soc. 1969, 91, 5644–5645;
- 2dK. Naumann, G. Zon, K. Mislow, J. Am. Chem. Soc. 1969, 91, 7012–7023;
- 2eK. L. Marsi, J. Org. Chem. 1974, 39, 265–267;
- 2fT. Coumbe, N. J. Lawrence, F. Muhammad, Tetrahedron Lett. 1994, 35, 625–628;
- 2gH. C. Wu, J. Q. Yu, J. B. Spencer, Org. Lett. 2004, 6, 4675–4678;
- 2hM. Berthod, A. Favre-Reguillon, J. Mohamad, G. Mignani, G. Docherty, M. Lemaire, Synlett 2007, 1545–1548;
- 2iC. Petit, A. Favre-Reguillon, B. Albela, L. Bonneviot, G. Mignani, M. Lemaire, Organometallics 2009, 28, 6379–6382;
- 2jC. J. O'Brien, J. L. Tellez, Z. S. Nixon, L. J. Kang, A. L. Carter, S. R. Kunkel, K. C. Przeworski, G. A. Chass, Angew. Chem. Int. Ed. 2009, 48, 6836–6839; Angew. Chem. 2009, 121, 6968–6971.
- 3P. Li, R. Wischert, P. Métivier, Angew. Chem. Int. Ed. 2017, 56, 15989–15992; Angew. Chem. 2017, 129, 16205–16208.
- 4
- 4aK. V. Rajendran, D. G. Gilheany, Chem. Commun. 2012, 48, 817–819;
- 4bK. V. Rajendran, D. G. Gilheany, Chem. Commun. 2012, 48, 10040–10042.
- 5H. Babad, A. G. Zeiler, Chem. Rev. 1973, 73, 75–91.
- 6
- 6aL. Horner, H. Hoffmann, P. Beck, Chem. Ber. 1958, 91, 1583–1588;
- 6bR. Salmon, Encyclopedia of Reagents for Organic Synthesis, Wiley, New York, 2001;
- 6cT. Yano, M. Hoshino, M. Kuroboshi, H. Tanaka, Synlett 2010, 801–803;
- 6dT. Yano, M. Kuroboshi, H. Tanaka, Tetrahedron Lett. 2010, 51, 698–701.
- 7Method for synthesizing cyclohexyl-substituted phosphines: A. Tishkov, K. Massonne, D. Mirk, J. Henkelmann, M. Maase, T. Wettling (BASF SE, Ludwigshafen), US 2010/0137643 A1, 2010.
- 8
- 8aD. W. Stephan, Org. Biomol. Chem. 2008, 6, 1535–1539;
- 8bD. W. Stephan, Dalton Trans. 2009, 3129–3136;
- 8cD. W. Stephan, G. Erker, Angew. Chem. Int. Ed. 2010, 49, 46–76; Angew. Chem. 2010, 122, 50–81;
- 8dJ. Paradies, Synlett 2013, 777–780;
- 8eD. Stephan, G. Erker, Isr. J. Chem. 2015, 55, 133–133;
- 8fD. W. Stephan, J. Am. Chem. Soc. 2015, 137, 10018–10032;
- 8gD. W. Stephan, G. Erker, Angew. Chem. Int. Ed. 2015, 54, 6400–6441; Angew. Chem. 2015, 127, 6498–6541.
- 9
- 9aC. B. Caputo, L. J. Hounjet, R. Dobrovetsky, D. W. Stephan, Science 2013, 341, 1374–1377;
- 9bL. J. Hounjet, C. B. Caputo, D. W. Stephan, Dalton Trans. 2013, 42, 2629–2635;
- 9cM. Perez, C. B. Caputo, R. Dobrovetsky, D. W. Stephan, Proc. Natl. Acad. Sci. USA 2014, 111, 10917–10921;
- 9dM. H. Holthausen, M. Mehta, D. W. Stephan, Angew. Chem. Int. Ed. 2014, 53, 6538–6541; Angew. Chem. 2014, 126, 6656–6659;
- 9eS. A. Weicker, D. W. Stephan, Bull. Chem. Soc. Jpn. 2015, 88, 1003–1016;
- 9fM. H. Holthausen, R. R. Hiranandani, D. W. Stephan, Chem. Sci. 2015, 6, 2016–2021.
- 10T. vom Stein, M. Perez, R. Dobrovetsky, D. Winkelhaus, C. B. Caputo, D. W. Stephan, Angew. Chem. Int. Ed. 2015, 54, 10178–10182; Angew. Chem. 2015, 127, 10316–10320.
- 11
- 11aJ. M. Blackwell, E. R. Sonmor, T. Scoccitti, W. E. Piers, Org. Lett. 2000, 2, 3921–3923;
- 11bM. Rubin, T. Schwier, V. Gevorgyan, J. Org. Chem. 2002, 67, 1936–1940;
- 11cJ. Hermeke, M. Mewald, M. Oestreich, J. Am. Chem. Soc. 2013, 135, 17537–17546;
- 11dA. Simonneau, M. Oestreich, Angew. Chem. Int. Ed. 2013, 52, 11905–11907; Angew. Chem. 2013, 125, 12121–12124;
- 11eL. Greb, S. Tamke, J. Paradies, Chem. Commun. 2014, 50, 2318–2320;
- 11fM. Oestreich, J. Hermeke, J. Mohr, Chem. Soc. Rev. 2015, 44, 2202–2220;
- 11gM. H. Holthausen, J. M. Bayne, I. Mallov, R. Dobrovetsky, D. W. Stephan, J. Am. Chem. Soc. 2015, 137, 7298–7301;
- 11hM. H. Holthausen, R. R. Hiranandani, D. W. Stephan, Chem. Sci. 2015, 6, 2016–2021;
- 11iM. Pérez, Z.-W. Qu, C. B. Caputo, V. Podgorny, L. J. Hounjet, A. Hansen, R. Dobrovetsky, S. Grimme, D. W. Stephan, Chem. Eur. J. 2015, 21, 6491–6500;
- 11jM. Mehta, M. H. Holthausen, I. Mallov, M. Perez, Z. W. Qu, S. Grimme, D. W. Stephan, Angew. Chem. Int. Ed. 2015, 54, 8250–8254; Angew. Chem. 2015, 127, 8368–8372;
- 11kM. Mehta, I. G. de la Arada, M. Perez, D. Porwal, M. Oestreich, D. W. Stephan, Organometallics 2016, 35, 1030–1035;
- 11lV. Fasano, J. H. W. LaFortune, J. M. Bayne, M. J. Ingleson, D. W. Stephan, Chem. Commun. 2018, 54, 662–665;
- 11mA. Augurusa, M. Mehta, M. Perez, J. T. Zhu, D. W. Stephan, Chem. Commun. 2016, 52, 12195–12198.
- 12
- 12aS. M. Godfrey, C. A. McAuliffe, R. G. Pritchard, J. M. Sheffield, Chem. Commun. 1996, 2521–2522;
- 12bN. Bricklebank, S. M. Godfrey, C. A. McAuliffe, P. Deplano, M. L. Mercuri, J. M. Sheffield, Dalton Trans. 1998, 2379–2382;
- 12cS. M. Godfrey, C. A. McAuliffe, I. Mushtaq, R. G. Pritchard, J. M. Sheffield, Dalton Trans. 1998, 3815–3818;
- 12dS. M. Godfrey, C. A. McAuliffe, R. G. Pritchard, J. M. Sheffield, Chem. Commun. 1998, 921–922.
- 13In chloroform the equilibrium was shifted to the chlorophosphonium species, wheras in toluene the dichlorophosphorane was the dominant species.
- 14
- 14aD. Naumann, H. Butler, R. Gnann, Z. Anorg. Allg. Chem. 1992, 618, 74–76;
- 14bL. Greb, C. G. Daniliuc, K. Bergander, J. Paradies, Angew. Chem. Int. Ed. 2013, 52, 5876–5879; Angew. Chem. 2013, 125, 5989–5992;
- 14cJ. A. Nicasio, S. Steinberg, B. Ines, M. Alcarazo, Chem. Eur. J. 2013, 19, 11016–11020;
- 14dS. Tussing, L. Greb, S. Tamke, B. Schirmer, C. Muhle-Goll, B. Luy, J. Paradies, Chem. Eur. J. 2015, 21, 8056–8059;
- 14eS. Tussing, K. Kaupmees, J. Paradies, Chem. Eur. J. 2016, 22, 7422–7426.
- 15
- 15aL. Goerigk, S. Grimme, J. Chem. Theory Comput. 2011, 7, 291–309;
- 15bB. M. Axilrod, E. Teller, J. Chem. Phys. 1943, 11, 299–300;
- 15cY. Muto, Proc. Phys. Math. Soc. Jpn. 1943, 17, 629–631;
- 15dA. Klamt, G. Schüürmann, J. Chem. Soc. Perkin Trans. 2 1993, 0, 799–805;
- 15eF. Eckert, A. Klamt, AIChE J. 2002, 48, 369–385;
- 15fF. Weigend, R. Ahlrichs, Phys. Chem. Chem. Phys. 2005, 7, 3297;
- 15gS. Grimme, J. Antony, S. Ehrlich, H. A. Krieg, J. Chem. Phys. 2010, 132, 154104;
- 15hS. Grimme, S. Ehrlich, L. Goerigk, J. Comput. Chem. 2011, 32, 1456–1465;
- 15iF. Furche, R. Ahlrichs, C. Hättig, W. Klopper, M. Sierka, F. Weigend, Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2014, 4, 91–100;
- 15jS. Grimme, A. Hansen, J. G. Brandenburg, C. Bannwarth, Chem. Rev. 2016, 116, 5105–5154;
- 15kS. Grimme, J. G. Brandenburg, C. Bannwarth, A. Hansen, J. Chem. Phys. 2015, 143, 054107;
- 15lTURBOMOLE, V7.0.2 ed., 2015, a Development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989–2007, TURBOMOLE GmbH, since 2007; Available from http://www.turbomole.com;
- 15mF. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2012, 2, 73–78;
- 15nF. Neese, V. 4.0.0 ed., MPI Für Chemische Energiekonversion, Mülheim A. D. Ruhr, Germany, 2017. ORCA: An Ab Initio, Density Functional and Semiempirical Program Package.
- 16
- 16aS. Grimme, C. Bannwarth, P. A. Shushkov, J. Chem. Theory Comput. 2017, 13, 1989–2009;
- 16bS. Grimme, C. Bannwarth, S. Dohm, A. Hansen, J. Pisarek, P. Pracht, J. Seibert, F. Neese, Angew. Chem. Int. Ed. 2017, 56, 14763–14769; Angew. Chem. 2017, 129, 14958–14964.
- 17S. Grimme, H. Kruse, L. Goerigk, G. Erker, Angew. Chem. Int. Ed. 2010, 49, 1402–1405; Angew. Chem. 2010, 122, 1444–1447.